COVID19 Vaccine
Jupiter Medical Center is not a testing site.
If you suspect you might be infected with COVID-19, please DO NOT visit the Emergency Department for a COVID-19 test. Instead seek out a community testing site, a home test kit or visit one of our Urgent Care locations:
Jupiter
5430 Military Trail, Suite 64 (Abacoa Shopping Center, next to McDonald’s)
1335 W. Indiantown Road (West of Delaware Blvd., next to Harmony Animal Hospital)
Palm Beach Gardens
3250 PGA Blvd. (Glass building – southeast corner of PGA Blvd. and Fairchild Gardens Ave.)
West Palm Beach
625 N. Flagler Drive (West side of Flagler Memorial Bridge)
Stuart
2628 S.E. Federal Highway (Baron Shoppes – west side of Federal Hwy., next to City Diner)
Jupiter Medical Center’s Emergency Department is prioritizing patients with medical conditions requiring emergency care and those with critical illness. We strongly discourage patients who are asymptomatic or have mild symptoms from coming to the emergency department so we may preserve resources for those in our community with emergent medical needs.
Effective March 23, 2022
Visiting Hours are from 8am to 8pm.
- All non-covid inpatients and outpatient procedural patients may have unrestricted visitors over the age of 5, during visting hours, loves ones may stay the night as long as the manager/director approves.
- This includes Peds, NICU, Cath Lab, inpatient and outpatient surgery, interventional radiology, GI lab and the cardiac cath lab.
- Emergency Department will allow 1 visitor after the patient is checked into a bed, for NON-COVID patients.
- Visitors may utilize the coffee shop in the East lobby, the cafeteria and the gift shop.
- All visitors must wear a mask.
Maternity Visitation
- During labor and delivery, all non-covid patients may have 2 adult visitors. No swap-outs allowed.
- Doulas are permitted and are not counted as one of the visitors.
- After delivery, all non-covid patients may have 2 visitors over the age of 5, during regular visiting hours. Swap-outs are permitted, with only 2 guests at one time. One adult may stay the night.
- Quiet hours are between 2pm - 4pm, and we ask that you do not visit during this time
- Visitors may utilize the Cafeteria, Coffee Shop, or Gift Shop
- A Covid test is required for all scheduled labor inductions and C-Sections. Your provider will provide you more details.
- A rapid Covid test may be ordered when you arrive in labor depending on certain circumstances.
- Patients with a positive Covid test are only permitted one visitor who will remain in the patient room throughout the stay.
CEO Update 3/31/2022
COVID hospitalizations continue to be at low levels, including Florida and Palm Beach County. A subvariant B.A.2 of the omicron strain has been proliferating and has become the most dominant strain of COVID in the US and globally. There continues to be a focus on monoclonal antibody therapy for appropriate patients. This week, the FDA and CDC released guidelines for a fourth booster dose in selected groups.
Florida Metrics
- There were 8,774 new cases during the week ending 3/24 (9% increase)
- The percent positive rate was 2.3% for the week ending 3/24 (0.4 percentage point increase)
- The Rt value is 1.0 (epiforecasts.io 3/28)
- 15.4 million individuals have received at least one vaccine dose (74% of population over age 5)
Palm Beach County Metrics
- There were 680 new cases during the week ending 3/24 (14% decrease over 2 weeks)
- The percent positive rate was 3.1% for the week ending 3/24 (increasing)
- 1.07 million individuals have received at least one vaccine dose (76% of population over age 5)
Jupiter Medical Center Metrics
- There are 2 Covid patients currently hospitalized
- There are no COVID patients in the ICU
- 196 patients (13% increase) were tested in our urgent care centers and 7% tested positive (1% increase)
COVID variants
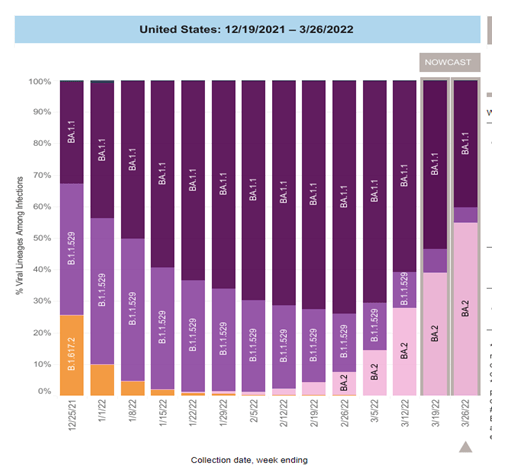
The B.A.2 subvariant of omicron has become the most common variant in the US and globally (Fig 1). The B.A.2 variant appears to be significantly more transmissible than the original omicron variant but does not appear to cause more severe illness. There has been an uptick in new cases associated with the increase in the B.A.2 variant in Europe and this has been accompanied by an increase in hospitalizations in some countries including the U.K. Thus far, the increase in the percentage of cases caused by B.A.2 in the U.S. has not been associated with a significant increase in cases or hospitalizations. Florida is in region 4 of the US (designated by the CDC), with 39.4% of new cases being caused by the B.A.2 variant.
Monoclonal Antibody Therapy
An important consequence of the shift in subvariants is a loss of efficacy of sotrovimab therapy. Consequently, the FDA is withdrawing its authorization of sotrovimab in areas of the U.S. where the B.A.2 variant predominates including New York, New Jersey and Massachusetts.
The FDA has given emergency approval to another monoclonal antibody, bebtelovimab, that does inhibit the original and the B.A.2 omicron subvariants. The treatment has been approved for treatment of mild to moderate COVID in individuals over 12 years of age with a positive COVID test who are at high risk for progression to severe disease.
EVUSHELD
EVUSHELD has been approved for pre-exposure prophylaxis in individuals with moderate to severe immune compromise that may not mount an adequate immune response to COVID vaccination or for those patients for whom vaccination with any available COVID vaccine is not recommended due to a history of a severe adverse reaction to a COVID vaccine or vaccine component. This is an important treatment for our immunocompromised patients. In the PROVENT trial, there was a 77% reduction in symptomatic COVID illness at 6 months in patients receiving EVUSHELD.
Vaccines
An important announcement was made this week regarding an additional booster
dose by the FDA and CDC. A second booster dose of Pfizer-BioNTech or the
Moderna vaccines was authorized for individuals over 50 years of age at least 4 months
after a first booster dose. In addition, a second booster dose is authorized
for patients who have had a prior solid organ transplant or an equivalent
level of immune compromise (Pfizer-BioNTech vaccine is authorized for
those patients over 12 years of age and Moderna is authorized for those
patients over 18 years of age). Dr Peter Marks MD, PhD of the FDA stated:
“Current evidence suggests some waning of protection over time against
serious outcomes from COVID-19 in older and immunocompromised individuals.
Based on an analysis of emerging data, a second booster dose of either
the Pfizer-BioNTech or Moderna COVID-19 vaccine could help increase protection
levels for these higher-risk individuals.”
As another season draws to a close in South Florida, there is cautious optimism that B.A.2 will not be associated with a significant surge.
Stay well.
Florida Metrics
- There were 198,719 new cases during the week ending 1/27 (30% decrease). The preponderance of these cases are occurring in the northern 1/3 of the state.
- The percent positive rate was 23.5% for the week ending 1/27 (12% decrease).
- The Rt value is 0.45 (covidestim.org 1/28).
- 15.19 million individuals have received at least one vaccine dose (73% of population over age 5).
Palm Beach County Metrics
- There were 8,741 new cases during the week ending 1/27 (68% decrease over 2 weeks).
- The percent positive rate was 19.7% for the week ending 1/27 (decreasing).
- 1.06 million individuals have received at least one vaccine dose (75% of population over age 5).
- The Rt values is 0.28 (covidestim.org).
Jupiter Medical Center Metrics
- There are 23 Covid patients currently hospitalized (50% decrease). 11 of these patients are unvaccinated, 9 have received their primary vaccine series, and 3 patients had received boosters.
- 2 Covid patients are in the ICU and both are on a ventilator.
- A total of 258 individuals (40% decrease) were tested at the JMC Urgent Care sites with a percent positivity rate of 26%.
Vaccination
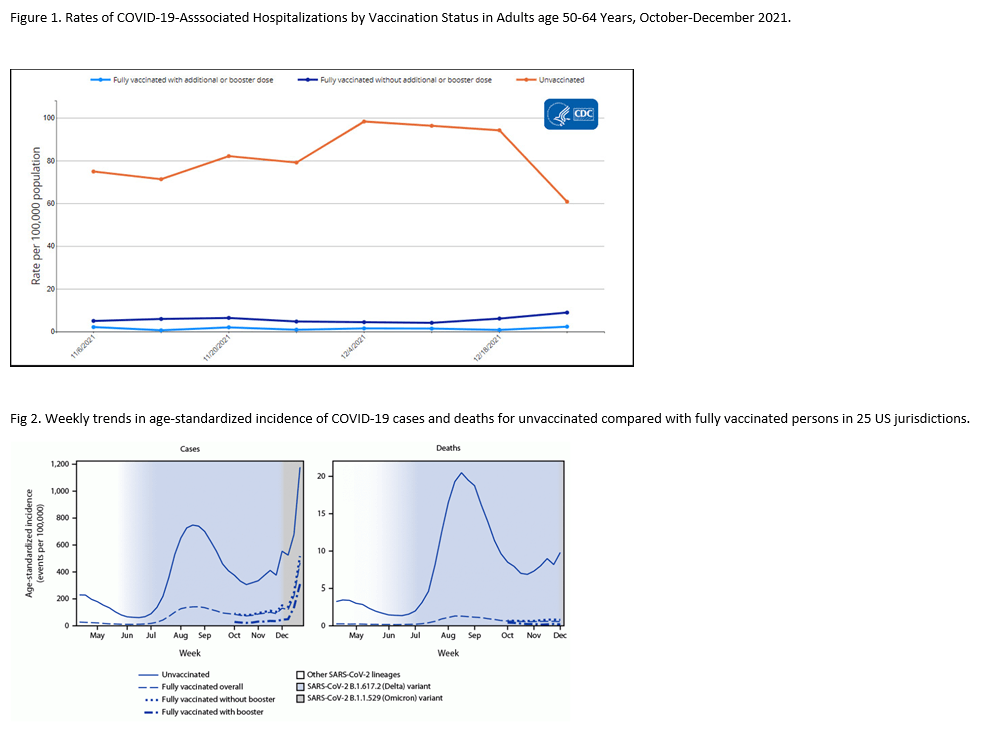
Evidence is continuing to accrue about the effectiveness of vaccination during the omicron phase of the COVID pandemic. In the current COVID Data Tracker Weekly Review put out by the CDC, they note the substantial protection offered both by vaccination and by full vaccination plus a booster dose against severe illness requiring hospitalization with data through December 25 (Fig 1). In addition, a recent report shows that compared to vaccinated adults, unvaccinated adults had more than 50 times the risk of COVID-associated death (Fig 2).
Therapy
Due to data demonstrating the lack of effectiveness of REGEN-COV and bamlanivimab/etesevimab against the omicron variant, the FDA has withdrawn its Emergency Use Authorization (EUA) for these therapies. As a result, the state monoclonal antibody therapy centers across Florida were closed last week.
Sotrovimab is the monoclonal antibody treatment that has retained effectiveness against the omicron variant and Jupiter Medical Center continues to offer sotrovimab therapy in collaboration with the medical staff.
Oral antiviral treatment is available with a physician’s prescription and the state continues to offer Paxlovid and/or molnupiravir at selected pharmacies. Our closest site is the Publix pharmacy located in the Crosstown Plaza on Military Trail in West Palm Beach. On January 31, the pharmacy indicated that they have exhausted their allocation of Paxlovid but continue to have a supply of molnupiravir. They have ordered additional Paxlovid, with no definite date of availability.
We are glad to see the rapid decline in omicron cases as we again strive to regain a sense of normalcy, while recovering from this most recent Covid surge.
Stay safe.
CEO Update 1/18/2022
Current data suggests that the Omicron surge is at or near its peak in Florida. Other states which had early Omicron surges are now seeing decreasing numbers of daily new cases (New York, New Jersey, and Massachusetts).
Florida Metrics
- There were 430,297 new cases during the week ending 1/13 (8.7% increase).
- The percent positive rate was 29.3% for the week ending 12/30 (6.4% decrease).
- The Rt value is 0.87 (covidestim.org 1/16).
- 15.07 million individuals have received at least one vaccine dose (72% of population over age 5).
Palm Beach County Metrics
- There were 26,918 new cases during the week ending 12/30 (8% increase over 2 weeks).
- The percent positive rate was 29.7% for the week ending 12/30 (increasing).
- 1 million individuals have received at least one vaccine dose (74% of population over age 5).
- The Rt values is 0.32 (covidestim.org).
Jupiter Medical Center Metrics
- There are 42 Covid patients currently hospitalized (16% decrease).
- 3 Covid patients are in the ICU and 1 is on a ventilator.
- A total of 717 individuals (43% decrease) were tested at the JMC Urgent Care sites with a percent positivity rate of 38%.
Omicron Surge
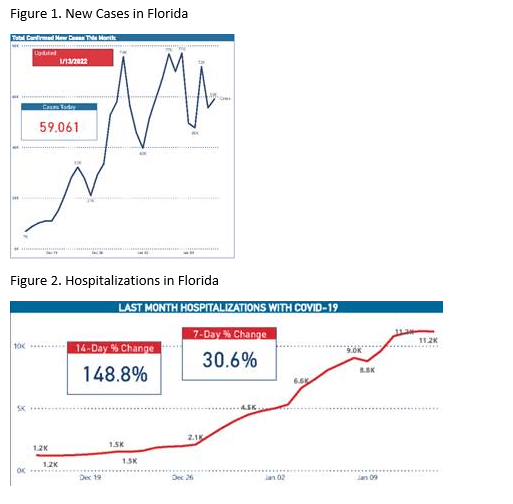
New cases and hospitalizations continue to increase in the US. However, several states that were in the early Omicron surge are already seeing reductions in daily cases. New cases and hospitalizations in Florida appear to be plateauing (Fig 1 and 2) and the reproduction number in Palm Beach County is reported as 0.35 on January 15 (less than 1 is associated with a surge reduction).
A recent study demonstrated that there was a high prevalence of asymptomatic infection in individuals shedding virus, which is undoubtedly a factor in the rapid dissemination of this variant. On the positive side, another U.S. study compared over 50,000 individuals infected with the Omicron variant to a group infected with Delta and found that there was a 53% reduced risk of hospitalization, a 74% reduced risk of ICU admission, and a 91% lower risk of death in the group infected with Omicron. This is consistent with data from South Africa and England which also suggest reduced virulence of this strain.
Vaccination
A recent study which included over 800,000 participants showed that individuals who received a booster dose at least 5 months after completion of their primary vaccine series had a 90% lower mortality from COVID than participants who did not receive a booster. In addition, there was an 83% reduction in the risk of developing a symptomatic COVID infection compared to the non-booster group. This large study clearly shows the benefit of booster doses in reducing both symptomatic infections and mortality related to COVID.
Therapeutics
Supplies of both monoclonal antibody therapies and oral antiviral treatments remain very limited. Last week JMC received a limited quantity of sotrovimab (the antibody therapy which is active against the omicron variant) and we have been collaborating with our medical staff to provide this therapy for appropriate patients.
Supplies of Paxlovid and molnupiravir (the oral antivital therapies) remain very limited. Fortunately, 10 million doses are expected to be delivered by June and the remainder by September. The Florida Department of Health is distributing their allocation of oral antiviral therapies to various pharmacies across the state (the pharmacies which have received allocations are listed on the floridahealthcovid19.gov website under the treatment locator tab). The therapy must be prescribed by an authorized healthcare provider.
Testing
The US government announced that individuals could begin ordering at-home COVID tests on Wednesday January 19th at COVIDtests.gov. The tests are expected to ship within 7-12 days of being ordered.
Data indicating that the omicron surges appear to be ebbing, coupled with the availability of antibody treatment and oral antiviral therapy for those afflicted, provides cause for optimism for the months ahead.
Stay safe.
CEO Update 1/4/2022
As we usher in the New Year, we are facing a dramatic increase in Covid cases resulting from the omicron variant. While evidence has accumulated that this variant is associated with less severe disease, the sheer increase in cases has resulted in a significant increase in hospitalizations. The large number of cases has also resulted in staff shortages due to illness across industries, including healthcare. In addition, the resistance of this variant to some of the monoclonal antibody therapies has diminished the utility of this treatment modality. While oral antiviral COVID therapies have recently been approved, supply is scarce, limiting access.
Florida Metrics
- There were 298,455 new cases during the week ending 12/30 (130% increase)
- The percent positive rate was 26.5% for the week ending 12/30 (90% increase)
- The Rt value is 3.27 (epiforecasts.io 1/1, increasing)
- 14.9 million individuals have received at least one vaccine dose (71% of population over age 5)
Palm Beach County Metrics
- There were 24,488 new cases during the week ending 12/30 (940% increase over 2 weeks)
- The percent positive rate was 30.6% for the week ending 12/30 (increasing)
- 1 million individuals have received at least one vaccine dose (74% of population over age 5)
- The Rt values is 1.24 (covidestim.org)
Jupiter Medical Center Metrics
- There are 37 Covid patients currently hospitalized (22 are unvaccinated; 15 are vaccinated with 4 having received boosters)
- 3 Covid patients are in the ICU, all requiring ventilators
- A total of 1,628 individuals were tested at the JMC Urgent Care sites with a percent positivity rate of 39%
Omicron Surge
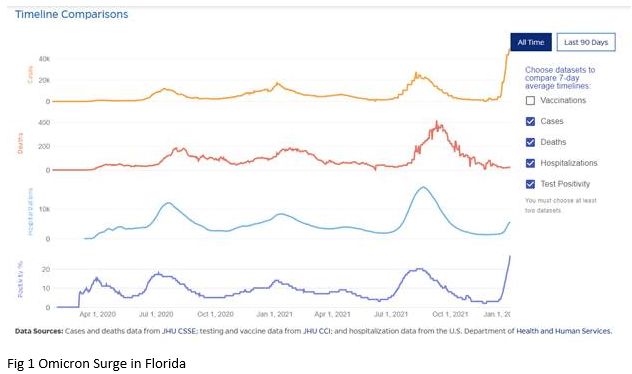
The current surge in cases has now surpassed the prior peak levels during the COVID pandemic (Fig 1), with 486,428 new cases being reported on December 29. The latest data from the CDC reveals that 95.4% of the Covid cases across the U.S. can be attributed to the omicron variant. Data from South Africa and England indicate that the omicron surge is likely to follow an inverted V shape. With the surge in South Africa lasting approximately 4 weeks, this offers hope for a rapid recovery. Various models predict that the peak of cases in Florida will occur somewhere between mid-January and mid-February.
Monoclonal antibody therapy
Regen-COV and bamlanivimab/etesevimab, which were used successfully in previous phases of the pandemic, do not appear to retain activity against the Omicron variant. In region 4 (as designated by HHS to include Florida, Alabama, Georgia, Kentucky, Tennessee, Mississippi and the Carolinas), the omicron variant now represents 97.3% of the Covid cases.
Sotrovimab, an antibody therapy that has demonstrated strong evidence of efficacy against omicron is now being allocated by U.S. health officials to states based on the number of Covid cases and hospitalizations.
Evusheld is an additional new monoclonal antibody therapy. It has received an EUA (Emergency Use Authorization) for pre-exposure prophylaxis in high-risk patients including those with moderate to severe immune compromise and those who have had severe allergic reactions to COVID vaccines. The PROVENT study in 5,000 patients demonstrated a 77% reduction in the development of COVID infections in the treatment group. This reduction in risk of infection was maintained for 6 months and importantly, a recent study demonstrated that Evusheld retained its neutralizing activity against the Omicron variant.
Florida state monoclonal antibody sites continue to schedule patients for monoclonal antibody therapy, expanding access and helping ease the burden on hospitals.
Oral Antiviral Therapy
A recent development in COVID therapy is the approval of Pfizer’s Paxlovid and Merck’s molnupiravir as oral therapy for early COVID disease in patients at risk of severe disease, such as those with weakened immune systems. They are designed to be taken at home within the first few days of contracting Covid-19. Early data indicates Paxlovid reduces the risk of hospitalization or death by 89% while molnupiravir reduces the risk by 30% and both appear to be effective against the omicron variant. DHS (Department of Health and Human Services) allocated 65,000 courses of Paxlovid and 300,000 courses of molnupiravir last week and these are being distributed through state health departments, which will decide the hospitals and pharmacies that will receive shipments. Many of these doses are in transit and have not yet reached their destinations. It is anticipated that the availability of these therapies will remain very limited for the upcoming weeks.
Vaccination
The FDA has authorized COVID booster shots for teens aged 12 to 15 years of age by extending the EUA for the Pfizer booster shot. The Advisory Committee for Immunization Practice of the CDC is scheduled to meet later this week and consider this authorization. The committee recommendation will then go to the CDC director for final approval. The FDA is basing this decision on data from Israel that demonstrated no significant safety concerns in this age group with no cases of myocarditis reported.
In addition, the FDA is moving the interval for booster doses from six to five months based on the Israeli data, which showed that the shorter interval is effective.
The next 4-6 weeks will pose significant challenges as the impact of the rapid rise in Covid cases is compounded by staffing shortages across industries. Fortunately, the decreased clinical severity of omicron coupled with the advent of new therapeutics will help us weather the current wave of the Covid-19 pandemic.
Best wishes for a safe, happy and healthy New Year.
Effective December 29, 2021
Patients are allowed 1 visitor (over the age of 18) per day. Visitation will not occur in rooms with COVID patients.
Visiting hours occur daily from 11am - 7pm
Visitors will enter via the East entrance. Labor & Delivery Patients/Significant others and Pediatric visitors will enter via the South entrance.
Inpatient visitation will resume as follows:
- INPATIENTS ONLY are allowed 1 visitor (over the age of 18) per day at the same time. Please use the East entrance.
- Visitors must present with a government issued ID and must wear a mask at all times.
- Visitors will be directed to the patient room and asked to stay in the room.
- Visitors may utilize the cafeteria but must wear a mask when not eating/drinking. Visitors may also purchase coffee at the Coffee Shop in the east lobby.
- Visitors will not be permitted entry after 6:30pm.
- Visitation will not occur in rooms with COVID patients.
-
Pediatric patients are allowed to have both parents visit at the same time.
One parent can spend the night.
- Pediatric visitors will enter via the South lobby.
- Labor & Delivery patients/significant others will enter using the South lobby and the significant other should plan to stay the entire encounter, there will be no switching out of visitors.
- NICU patients may have both parents visit at the same time.
- Emergency Department (ED) patients may have 1 visitor, once placed in a room in the ED.
-
INPATIENT and OUTPATIENT Surgical patients (this does NOT include patients
here for procedures such as cardiac caths, eletrophysiology, GI lab or
interventional radiology):
- Will be permitted 1 visitor to accompany them to the preop area.
- Visitor must present their government issued ID and must wear a mask at all times.
- Visitors will be permitted to stay with their loved one in the preop area.
- Visitors may utilize the cafeteria but must wear a mask when not eating/drinking. Visitors may purchase coffee at the Coffee Shop in the East lobby.
- Visitors may wait for their loved one in the surgical waiting area and must wear a mask at all times.
Effective December 6, 2021
Visiting hours occur daily from 11am - 7pm
Visitors will enter via the East entrance. Labor & Delivery Patients/Significant others and Pediatric visitors will enter via the South entrance.
Inpatient visitation will resume as follows:
- INPATIENTS ONLY are allowed 2 visitors (over the age of 18) per day at the same time. Please use the East entrance.
- Visitors must present with a government issued ID and must wear a mask at all times.
- Visitors will be directed to the patient room and asked to stay in the room.
- Visitors may go to the cafeteria but must wear a mask when not eating/drinking. Visitors may also purchase coffee at the Coffee Shop in the east lobby.
- Visitors will not be permitted entry after 6:30pm.
- Visitation will not occur in rooms wtih COVID patients.
-
Pediatric patients are allowed to have both parents visit at the same time.
One parent can spend the night.
- Pediatric visitors will enter via the South lobby.
- Labor & Delivery patients/significant others will enter using the South lobby and the significant other should plan to stay the entire encounter, there will be no switching out of visitors.
- NICU patients may have both parents visit at the same time.
- Emergency Department (ED) patients may have 1 visitor, once placed in a room in the ED.
-
INPATIENT and OUTPATIENT Surgical patients (this does NOT include patients
here for procedures such as cardiac caths, eletrophysiology, GI lab or
interventional radiology):
- Will be permitted 1 visitor to accompany them to the preop area.
- Visitor must present their government issued ID and must wear a mask at all times.
- Visitors will be permitted to stay with their loved one in the preop area.
- Visitors may go to the cafeteria but must wear a mask when not eating/drinking. Visitors may purchase coffee at the Coffee Shop in the East lobby.
- Visitors may wait for their loved one in the surgical waiting area and must wear a mask at all times.
CEO Update 12/21/21
The Omicron variant is dominating current news regarding the Covid pandemic. According to estimates released by the CDC yesterday, the Omicron Covid-19 variant now accounts for approximately 75% of new coronavirus cases in the Unites States. Increased transmissibility as well as a genetic profile that may reduce its susceptibility to vaccination and monoclonal antibody therapy are cause for concern.
New England and the Midwest are seeing the impact of dual surges consisting of delta and omicron variants simultaneously. Hospitals in some areas were already seeing increased numbers of cases due to the Delta variant and now New York/New Jersey are also seeing increased numbers of omicron cases, with up to 13% of new cases being omicron last week. Given the rapid doubling time of the omicron variant, it is likely that the overall case numbers will continue to surge.
Florida Metrics
- There were 29,568 new cases during the week ending 12/16 (120% increase)
- The percent positive rate was 5.4% for the week ending 12/16 (108% increase)
- The Rt value is 1.1 (epiforecasts.io 12/18, increasing)
- 14.6 million individuals have received at least one vaccine dose (70% of population over age 5)
Palm Beach County Metrics
- There were 2,445 new cases during the week ending 12/16 (160% increase)
- The percent positive rate was 6.5% for the week ending 12/16 (150% increase)
- 1 million individuals have received at least one vaccine dose (73% of population over age 5)
- The Rt values is 1.6 (covidestim.org)
Jupiter Medical Center Metrics
- There are 3 Covid patients currently hospitalized
- 2 Covid patients are in the ICU with one requiring a ventilator
- We tested 635 patients (up 82%) for Covid last week in our Urgent Care centers with a percent positive rate of 21% (increasing)
Omicron
Since its discovery in Botswana on November 11 and South Africa on November 12, the omicron variant is now present in 70 countries and in 45 states in the U.S. Two areas of concern are its increased transmissibility and its decreased susceptibility to current vaccination and monoclonal antibody therapy. This variant has over 30 mutations in the spike protein and several of these mutations are known to increase transmissibility. The omicron variant has spread rapidly in South Africa and in England with doubling times of 1-3 days (Fig 1).
In South Africa, cases increased by 10% within 24 hours. Early analysis has demonstrated that reinfections are 2.39 times more common with omicron than prior variants (study posted on December 2). A study from England estimated the reinfection rate of Omicron is 5.4 times that of Delta.
Vaccination/boosters
Breakthrough cases in vaccinated individuals are being seen more commonly with omicron. In a recent study, there was a significant reduction in neutralizing activity of blood from vaccinated or previously infected individuals against omicron. In a study prepublished in medRxiv from Massachusetts General Hospital, most individuals vaccinated with mRNA vaccines had undetectable neutralization of omicron. Fortunately, a booster dose induced potent neutralization of the omicron variant. Moderna also released data showing that the omicron variant was 49-84 times less sensitive to neutralization by their 2-shot regimen than the beta variant. However, a third dose induced a 12.6 fold increase in neutralization titers against omicron (posted December 15). A recent study from England demonstrated that 2 doses of the Pfizer or AstraZeneca vaccines provided limited or no protection against symptomatic disease with omicron. A booster with the Pfizer vaccine significantly increased protection (MedRxiv, December 14).
Both Pfizer and Moderna are already developing mRNA vaccines against the omicron variant. The CEO of Pfizer estimated that it would take 100 days to develop a vaccine against omicron using the mRNA platform. They had previously developed vaccines which targeted the beta and delta variants but decided against EUA (Emergency Use Authorization) applications because of the continued effectiveness of the existing vaccine against those variants.
Therapeutic agents
Omicron was recently demonstrated to be less susceptible to casirivimab and imdevimab, two of the available monoclonal antibody treatments (medRxiv, December 8).
Two oral agents are pending approval for EUAs from the FDA and the CDC. These agents are effective when administered within the first few days of a COVID infection. Paxlovid from Pfizer, which is a protease inhibitor, should maintain its effectiveness in patients infected with omicron. Likewise, molnupiravir, which blocks viral polymerase and inhibits multiplication of the virus should also maintain its effectiveness against the omicron variant. Molnupiravir has already been approved by an FDA advisory panel and is expected to receive full approval in the coming weeks.
Fortunately, the omicron variant appears to produce less severe disease than prior variants. The initial cases in South Africa were described in young university students. The symptoms included extreme fatigue, muscle aches, a scratchy throat and dry cough. Most of the patients did not require hospital admission. Since the initial reports, there has been a decoupling between the rapid rise in cases and a much lower increase in hospitalizations in South Africa.
In Palm Beach County, case numbers and the reproduction number (Rt) are rising while hospitalizations remain flat. This may be an indication that the increase in case numbers is related to the omicron variant, which at this time is believed to have a less severe clinical course. However, we must be prepared that this may represent a lag between the surge in cases and a subsequent increase in hospitalizations. We believe that the first explanation is the more likely one but will continue to monitor the data closely in the upcoming days and weeks.
Regrettably, the emergence of the omicron variant has suddenly altered hopes and plans for the upcoming season for many of us.
Best wishes for a safe and healthy holiday for you and your families.
CEO Update 11/10/21
New COVID cases and percent positivity continue to drop in Florida and Palm Beach County. There is also promising news regarding therapeutic agents for COVID. In the vaccination arena, the CDC has expanded its recommendations for vaccination to the 5-11 age group.
Statewide Metrics
- There were 11,069 new cases during the week ending 11/4 (14% decrease)
- The percent positive rate was 2.6% for the week ending 11/4 (13% decrease)
- The Rt value is 0.513 (covidestim.org 11/8)
- 14 million individuals have received at least one vaccine dose (73% of population over age 12)
Palm Beach County Metrics
- There were 793 new cases during the week ending 10/14 (decreasing)
- The percent positive rate was 2.9% for the week ending 10/14 (decreasing)
- 985,000 individuals have received at least one vaccine dose (76% of population over age 12)
Jupiter Medical Center
- There are 9 COVID patients currently hospitalized
- 1 patient is in the ICU and is on a ventilator
- We tested 175 patients for COVID this week in our Urgent Care centers (down 20%)
Therapeutics
Pfizer reported encouraging results from the phase 2/3 trial EPIC-HR of its oral agent Paxlovid. There was an 89% reduction in the risk of COVID hospitalization or death in this randomized, double-blind study in non-hospitalized COVID patients treated within the first 3 days of symptom onset compared to control patients. The decrease was 85% when patients received the drug within 5 days. Due to the excellent efficacy of the treatment, the study was stopped and Pfizer plans to use this data to support an EUA (Emergency Use Authorization) application.
Merck and Ridgeback Biotherapeutics recently submitted an EUA application for another oral antiviral agent molnupiravir. The Phase 3 MOVe-OUT trial in high-risk non-hospitalized COVID-positive patients with mild to moderate COVID-19 with symptom onset within 5 days of enrollment showed a 50% reduction in hospitalization or death. An independent Data Monitoring Committee recommended that the study be stopped early due to the positive results. The EUA application was filed on October 11.
Regeneron has reported that a single dose of its antibody cocktail RegenCOV reduced the risk of contracting COVID by 81.6% in the 2-8 month period following administration. This is especially significant in patients who are immunocompromised or those unresponsive to vaccination. The study participants were in the same household as a patient diagnosed with COVID within the prior 4 days. The participants received RegenCOV versus placebo. There were no hospitalizations in the treatment arm.
Vaccine
The FDA advisory committee met on October 26 and unanimously recommended that the FDA grant EUA for the COVID vaccine for children 5 to 11 years old after reviewing the safety and effectiveness of the vaccine in that age group. This was followed on October 29 by the FDA authorizing the emergency use of the COVID vaccine in 5 to 11 years old children using a 10 microgram dose given 3 weeks apart. On November 2, after a systematic review of available data, CDC’s ACIP (Advisory Committee on Immunization Practices) also made a recommendation for use of the Pfizer vaccine in children aged 5-11 years old.
We are pleased that the trend of COVID cases in our region continues to be favorable.
CEO Update 10/19/21
New COVID cases and percent positivity continue to decline in our region.
Florida Metrics
- There were 19,519 new cases during the week ending 10/14 (24% decrease)
- The percent positive rate was 3.8% for the week ending 9/30 (21% decrease)
- The Rt value is 0.51 (covidestim.org 10/18)
- 13.8 million individuals have received at least one vaccine dose (72% of population over age 12)
Palm Beach County Metrics
- There were 1,430 new cases during the week ending 10/14 (decreasing)
- The percent positive rate was 4.1% for the week ending 10/14 (decreasing)
- 973,000 individuals have received at least one vaccine dose (75% of population over age 12)
Jupiter Medical Center Metrics
- There are 9 COVID patients currently hospitalized (59% decrease over 2 weeks)
- 1 patient is in the ICU and is on a ventilator
Vaccines
The FDA vaccine advisory panel met twice last week. On day 1, they unanimously approved a booster of the Moderna vaccine for administration at least 6 months after the second vaccination. The booster dose would be 50 micrograms, which is half of the first shot dose. The groups recommended for booster include:
- people over age 65
- individuals 18 to 64 years old at high risk for severe COVID disease
- those with institutional or occupational exposure to COVID such as healthcare workers
On day 2, the panel considered a booster dose for the J+J vaccine. The panel unanimously voted for a second vaccine dose at least 2 months after the initial dose for all recipients of the J+J vaccine. Some of the speakers characterized this as a belief that the data indicates that the J+J vaccine should have also been a 2-shot vaccine based on the results that demonstrated a significantly lower effectiveness in preventing serious illness 4 months after vaccination.
During this meeting, the panel also considered “mix and match” vaccination, in which the individual receives a different type of vaccine from the initial vaccination. Research was presented by a US MixNMatch trial at 10 clinical sites consisting of 450 patients who received booster doses. Findings revealed that mixing boosters were safe without any increased adverse events. Booster doses of all 3 vaccine types led to increased antibody levels in participants who had received any of the 3 vaccine types initially. Heterologous boosters (a different vaccine type for the booster) generated higher responses than homologous boosters. mRNA vaccine boosters generated higher antibody titers than the J+J vaccine in the first 28 days after the boost. However, the panel did not make a recommendation about regarding “mix and match” vaccination and cited a lack of sufficient data to propose a recommendation at this time.
The next step in the process is a meeting of the Advisory Committee for Immunization Practice on Wednesday and Thursday of this week. COVID vaccination is on the agenda for Thursday, and it is likely that the Committee will recommend boosters for both Moderna and J+J vaccinated individuals. The Moderna booster will likely be recommended for the same groups as were identified for the Pfizer boosters. The J+J booster will be recommended for all persons over age 18 who received single dose vaccination with the J+J vaccine. It is expected that the acting commissioner of the FDA and the CDC director will issue recommendations by the end of this week.
Influenza Vaccination
We experienced a very mild flu season last year. The rate of flu hospitalization was only 4 per 100,000 compared to a usual rate of 70 per 100,000. Unfortunately, it is predicted that the flu season will be more severe this year.
The CDC and ACIP recommend flu vaccination for all persons over 6 months of age who do not have contraindications. Flu vaccination should ideally be administered by the end of October.
Individuals over age 65 and those with immunocompromise should consider a high dose flu vaccine.
Flu vaccines can be administered at the same time as a COVID vaccine but should be given at a different injection site (i.e., in opposite arms or in the same arm at least one inch apart).
Therapy
A meeting of the FDA advisory panel is scheduled for November 30 to consider Merck and Ridgeway Biotherapeutics application for an EUA of their oral therapeutic agent molnupiravir. Given the very promising results seen in the randomized trial with a 50% reduction in hospitalizations and deaths (primary endpoint), the FDA could also consider activating an expanded access protocol, which was done for convalescent plasma last year before an EUA was issued.
As a result of the continued favorable trend of Covid-19 cases in Florida coupled with the approval of vaccine boosters and the increased availability of therapeutics for early-stage Covid infection, we look forward to a happy and healthy holiday season.
Effective October 11, 2021
Visiting hours occur daily from 12pm - 6pm
Visitors will enter via the East entrance. Labor & Delivery Patients/Significant others and Pediatric visitors will enter via the South entrance.
Inpatient visitation will resume as follows:
- INPATIENTS ONLY are allowed 2 visitors (over the age of 18) per day at the same time. Please use the East entrance.
- Visitors must present with a government issued ID and must wear a mask at all times.
- Visitors will be directed to the patient room and asked to stay in the room.
- Visitors may go to the cafeteria but must wear a mask when not eating/drinking. Visitors may also purchase coffee at the Coffee Shop in the east lobby.
- Visitors will not be permitted entry after 5:30pm.
- Visitation will not occur in rooms wtih COVID patients.
-
Pediatric patients are allowed to have both parents visit at the same time.
One parent can spend the night.
- Pediatric visitors will enter via the South lobby.
- Labor & Delivery patients/significant others will enter using the South lobby and the significant other should plan to stay the entire encounter, there will be no switching out of visitors.
- NICU patients may have both parents visit at the same time.
- Emergency Department (ED) patients may have 1 visitor, once placed in a room in the ED.
-
INPATIENT and OUTPATIENT Surgical patients (this does NOT include patients
here for procedures such as cardiac caths, eletrophysiology, GI lab or
interventional radiology):
- Will be permitted 1 visitor to accompany them to the preop area.
- Visitor must present their government issued ID and must wear a mask at all times.
- Visitors will be permitted to stay with their loved one in the preop area.
- Visitors may go to the cafeteria but must wear a mask when not eating/drinking. Visitors may purchase coffee at the Coffee Shop in the East lobby.
- Visitors may wait for their loved one in the surgical waiting area and must wear a mask at all times.
CEO Update 10/5/21
We are pleased to report that Covid cases continue to trend favorably.
Statewide Metrics
- There were 37,772 new cases during the week ending 9/30 (33% decrease)
- The percent positive rate was 6.5% for the week ending 9/30 (24% decrease)
- The Rt value is 0.50 (covidestim.org 10/1)
- 13.6 million individuals have received at least one vaccine dose (71% of population over age 12)
Palm Beach County Metrics
- There were 2,416 new cases during the week ending 9/30 (decreasing)
- The percent positive rate was 6.5% for the week ending 9/16 (decreasing)
- 950,700 individuals have received at least one vaccine dose (74% of population over age 12)
Jupiter Medical Center
- There are 22 COVID patients currently hospitalized (decreasing)
- 5 patients are in the ICU with 2 requiring ventilators and 3 requiring BiPAP
- We tested 262 patients for COVID this week in our Urgent Care centers (down 10%)
Vaccines
The FDA has scheduled three meetings of its independent vaccine advisory committee for October. The first meeting on October 14 will review data regarding booster shots for the Moderna vaccine. The second meeting on October 15 will discuss J+J vaccine boosters and will also cover the administration of booster dose of a different vaccine than the original vaccination type (referred to as mix-and-match boosters). The third meeting on October 26 will involve Pfizer’s request for an EUA for its vaccine for 5- to 11-year-old children. The recommendations by the vaccine advisory committee are usually the last step before the FDA makes its formal recommendation. This is typically followed by recommendations from the Advisory Committee on Immunization Practices (ACIP) and CDC guidelines regarding vaccination.
J + J has presented data to the FDA from a range of studies prior to next week’s FDA meeting. The single shot vaccine produced a humoral and cellular immune response that lasted at least 8 months. The ENSEMBLE trial showed that the single shot vaccine was 85% effective against severe disease. A booster dose given 2 months after the initial dose resulted in a 4-fold increase in antibodies and was 94% effective in preventing mild to severe COVID infections. If the booster dose was given at an interval of 6 months, a 12-fold increase in antibody levels was seen.
In a study of over 3.4 million individuals which was published on October 4, new data was presented regarding the Pfizer vaccine effectiveness over 6 months. For fully vaccinated people over the entire study period, effectiveness against infection was 73% and against hospitalization was 90%. Effectiveness against infections fell from 88% during the first month after vaccination to 47% after 5 months. For infections caused by Delta variant, vaccine effectiveness against infection fell from 93% to 53% at 4 months after vaccination. Protection from hospitalization for Delta infections (genome sequenced) remained high at 93% for 6 months. This data is consistent with other information showing a decrease in protection from infection but a persistent effectiveness against severe disease by mRNA vaccines.
The CDC has also begun reporting on COVID vaccine effectiveness. There are a number of systems being used to monitor effectiveness in terms of infections, hospitalizations, and deaths in different groups. These include long-term care residents; healthcare providers and first responders; and hospitalized individuals.
Therapy
AstraZeneca has filed a request for an EUA for a long-acting antibody combination for prophylaxis of symptomatic COVID 19 which is given intramuscularly. The drug is a combination of two antibodies (tixagevimab and cilgavimab) derived from B cells donated by convalescent patients after COVID infection. In a PROVENT pre-exposure prophylaxis trial in 5,197 patients, the treatment reduced the risk of developing symptomatic COVID-19 by 77% compared to placebo. There were no cases of severe COVID or death in those treated with the study drugs.
Merck and Ridgeback Biotherapeutics have reported promising results in a Phase III trial of their oral COVID agent molnupiravir. In a study of 1,400 patients with risk factors for severe disease, a 5-day course of the drug given within 5 days of symptom onset, reduced hospitalization by 50%. In addition, there were no deaths in the patients who had received the study drug compared to 8/377 patients in the control group. This is an exciting development in the treatment of COVID, because the other therapies found to be effective early in the disease (monoclonal antibody agents) require intravenous infusions.
Holiday Travel
As many of us begin planning holiday travel, additional studies are being published about airline travel safety. These studies include analysis of actual flights and case studies, as well as ones using modeling to define risks and develop tactics to reduce risks. It should be stressed that the risk of onboard spread appears to be very small.
Some practical guidance which can be considered:
- In-flight mealtime in which most passengers have their masks off is a time of increased risk. A recent study found a 60% increased risk of transmission during a one-hour mealtime on a 12- hour flight. It would be prudent to consider keeping your mask on during meal service.
- A second period of increased risk is during boarding and deplaning. This is possibly related to people clustering and breathing in close proximity. One airline has instructed their pilots to leave ventilation systems running during boarding and deplaning. Being with a large group in a poorly ventilated jet bridge should also be avoided if possible.
- There is data which demonstrates that masking does reduce the risk of infection. Wearing a surgical mask or even an N-95 mask should be considered.
- If the toilet is used, your mask should be kept on. In addition, the lid should be closed prior to flushing. The flushing has been shown to create an upward air current with droplets being carried to a height of 100 cm.
- Frequent hand disinfection and wiping down surfaces such astray tables and arm rests are advised.
While the data is not conclusive, this is a list of activities which appear to play a role in transmission.
We continue to remain optimistic regarding the upcoming season in South Florida.
CEO Update 9/21/21
Nationally, headlines related to the COVID pandemic have been dominated by the FDA and CDC recommendations regarding booster doses in the U.S. In Florida, the continued reduction in new cases and percent positivity is welcome news.
Statewide Metrics
- There were 75,906 new cases during the week ending 9/16 (24% decrease)
- The percent positive rate was 11.2% for the week ending 9/16 (18% decrease)
- The Rt value is 0.51 (covidestim.org 9/19)
- 13.4 million individuals have received at least one vaccine dose (70% of population)
Palm Beach County Metrics
- There were 4,568 new cases during the week ending 9/16 (decreasing)
- The percent positive rate was 10% for the week ending 9/16 (decreasing)
- 950,700 individuals have received at least one vaccine dose (73% of population over 12)
Jupiter Medical Center
- There are 34 COVID patients currently hospitalized (17% decrease over the past 2 weeks)
- 7 patients are in the ICU with 3 requiring ventilators and 3 requiring BiPAP
- We tested 292 patients for COVID last week in our Urgent Care centers (down 40%) and the percent positive rate was 11% (decreasing)
Booster Doses
The FDA Vaccines and Related Biological Products Advisory Committee voted unanimously to recommend a third dose of the Pfizer-BioNTech vaccine to people 65 years and older, health care workers and others at high risk of occupational exposure, as well as people who are at risk of severe COVID. However, the committee voted against a proposal to recommend a third Pfizer dose for all individuals 16 and over. The panelists indicated that majority of vaccinated people have sufficient protection against severe illness requiring hospitalization or death.
Multiple studies were reviewed by the FDA Vaccine Advisory Committee. A study published Friday Sept 17 in MMWR looked at COVID-19 cases, hospitalizations, and Deaths by Vaccination Status in 13 U.S. jurisdictions from April through July 17, 2021. A key finding was that vaccination has been highly effective in preventing infection, hospitalization, or death even during the Delta surge (Fig 1).
Protection from hospitalization and death continued to be robust during the period of the study (Fig 2). However, researchers did see a higher-than-expected number of COVID infections in vaccinated individuals during the later period in the study, which would be consistent with a decrease in vaccine effectiveness from a 90% to an 80% level.
Additionally, the increases in the percentages of hospitalizations and deaths among vaccinated persons over 65 years of age were greater than were expected. This finding is consistent with a more significant reduction in vaccine effectiveness over time in the elderly.
Multiple other studies were considered by the committee. Several studies related to boosters have originated from Israel. In July, Israel reported a decline in vaccine effectiveness to 40% in preventing symptomatic infection but found that the vaccine was still 88% effective in preventing hospitalizations and deaths. Breakthrough infections were more common in individuals over the age of 60. In early July, Israel began giving booster doses to immunocompromised and transplant patients as they saw a spike in cases related to the Delta variant. Subsequently, Israel began giving booster doses in individuals over 60 years of age. Over a several week period, this decision was expanded to all persons over the age of 12. Following that decision, researchers found a significant improvement in protection which was present within 10 days following a third dose of the Pfizer vaccine. A third dose provided four times the protection against infection vs. 2 doses in people over the age of 60. The booster shots improved protection against serious illness and hospitalization by 5 to 6 times. Pfizer also presented data that their vaccine effectiveness against infection steadily decreases over time, to about 84% for vaccinated people 4 months after receiving the second dose. A recent study from the Mayo Clinic found that vaccine effectiveness against hospitalization remained high in July (Moderna, 81%; Pfizer 75%) but was lower against infection Moderna, 76%, Pfizer, 42%). A recent Oxford University study found that 90 days after the Pfizer vaccine, its effectiveness against preventing infections had dropped to 75%.
A meeting is scheduled this week for the Advisory Committee on Immunization Practice, which advises the CDC on whether it should recommend vaccines that the FDA has approved. ACIP will consider the recommendations made by the FDA vaccine committee last week and make a recommendation to the CDC.
Pediatric Vaccines
Earlier this week, Pfizer announced that a trial in children aged 5 to 11 demonstrated that their vaccine given as a two-dose regimen 3 weeks apart was safe and effective in generating a robust immune response. The dose was about one-third of the adult dose. The company plans to submit the data as soon as possible to the FDA for consideration of approval in this age group. It is projected that the review by the FDA will take between 4 and 6 weeks and that an approval could come by the end of October.
Vaccine Mandate for Healthcare Workers
On September 9, 2021 President Biden announced vaccine mandates for workers in healthcare settings that receive Medicare or Medicaid reimbursement, including, but not limited to hospitals, dialysis facilities, ambulatory surgical settings, and home health agencies. CMS (Centers for Medicare and Medicaid Services) is developing an Interim Final Rule containing pertinent details that will be issued in October.
We are glad to see a continued reduction in Covid-19 cases and hospitalizations as the upcoming season approaches.
CEO Update 9/7/21
The most significant news locally is the 20% week over week reduction in new Covid-19 cases and hospitalizations in Palm Beach County. Cases in the pediatric population continues to be an area of focus, as we wait for additional data about pediatric vaccinations to become available.
Statewide Metrics
- There were 129,240 new cases during the week ending 9/2 (20% decrease).
- The percent positive rate was 15.2% for the week ending 9/2 (23% decrease).
- The Rt value is 0.65 (covidestim.org 9/5).
- 13.1 million individuals have received at least one vaccine dose (69% of population).
Palm Beach County Metrics
- There were 6,863 new cases during the week ending 9/2 (decreasing).
- The percent positive rate was 12.6% for the week ending 9/2 (stable).
- 931,000 individuals have received at least one vaccine dose (72% of population over 12).
Jupiter Medical Center
- There are 41 COVID patients currently hospitalized (27% decrease over the past two weeks).
- 10 patients are in the ICU with 9 requiring ventilators and 1 requiring BiPAP.
- We tested 584 patients for COVID last week in our Urgent Care centers (down 27%) and the percent positive rate was 23% (decreasing).
Delta Surge
Palm Beach County and Jupiter Medical Center are seeing significant and rapid reductions in the number of new cases and new hospital admissions over the past 2 weeks. The percent positivity has also started to drop and the reproduction number (Rt) has been dropping rapidly. These forward-looking indicators suggest that we will continue to see substantial improvements in these parameters over the next few weeks.
Booster Doses
The data clearly demonstrates a reduction in effectiveness at preventing COVID infection with time as antibody levels decrease post vaccination. Since HHS (U.S. Department of Health and Human Services) initially released the recommendation for booster doses in all Americans receiving either the Pfizer or Moderna vaccine pending FDA and CDC’s ACIP (Advisory Committee on Immunization Practices) approval, several events have occurred. Two leading FDA scientists, the director and deputy director of the Office of Vaccines Research and Review, announced that they were leaving the agency due to their concerns that the usual process of having the FDA examine the data and make recommendations was not being followed. Dr Fauci subsequently stated that only the Pfizer vaccine may get FDA and CDC approval in time for the September 20 rollout and that Moderna will require additional time. Pfizer has already submitted their booster data to the FDA for review. There has been no announcement about the J+J vaccine, which produced lower initial levels of protection from COVID infection. It has been announced that an FDA advisory panel will review Pfizer’s application for a booster on September 17.
Pediatric Cases
The impact of the Delta surge on cases in children accompanied by the school year opening is being monitored and the CDC released several reports last week related to this subject. One study demonstrated that hospitalizations were 10x lower in vaccinated adolescents as compared to those who were unvaccinated. While mortality rates are very low in the pediatric age groups, about 25% of children require ICU level care if they require admission for COVID infection. The overall hospitalization rates for COVID infection in children were similar in the time periods before and during the Delta surge, suggesting that Delta did not produce more severe infection in the pediatric population. A second study demonstrated that ED visits and hospitalizations were significantly higher in states with lower population vaccination coverage and lower in states with higher vaccination coverage.
An additional study looked at a school outbreak in California. It was found that an unvaccinated teacher continued to come to school for 2 days following the development of COVID symptoms. The teacher at times read aloud to the students while not wearing a mask. 50% of 24 students developed COVID infections. 80% of students in the first 2 rows became ill, while only 28% of students in the next 3 rows developed infection. The authors concluded that in addition to vaccination, implementation of multimodal prevention strategies including masking, distancing, testing, ventilation, and staying home when symptomatic improves the safety of school instruction.
It is encouraging that the trend of Covid cases and hospitalizations continues to improve. We will provide further details regarding booster vaccinations as they become available.
CEO Update 8/25/21
The number of hospitalizations reported this week by the Florida Hospital Association has stabilized. We are currently seeing a downtrend in cases at JMC. Our Covid-19 cases reflect a 5% drop in our urgent care centers and a 15% drop in hospitalizations at JMC. The site covidestim.org and Scott Gottlieb, the former FDA commissioner are both reporting a reproduction number (Rt) of less than 1, which would indicate a decreasing number of cases moving forward. These facts support that we are at or just past the peak of the current surge. One important factor which could impact this trend was the recent opening of schools on August 10. There has been an uptrend in cases in the pediatric age ranges over the past several weeks, although there are currently no pediatric patients admitted with Covid at JMC.
Statewide Metrics
- There were 150,118 new cases during the week ending 8/19 (stable)
- The percent positive rate was 19.8% for the week ending 8/13 (up slightly)
- The Rt value is 0.99 (covidestim.org 8/13)
- 12.7 million individuals have received at least one vaccine dose (65% of population)
Palm Beach County Metrics
- There were 9,257 new cases during the week ending 8/19 (stable)
- The percent positive rate was 17.9% for the week ending 8/1 (stable)
- 905,000 individuals have received at least one vaccine dose (70% of population over 12)
Jupiter Medical Center
- There are 76 COVID patients currently hospitalized (15% decrease)
- 18 patients are in the ICU with 6 requiring ventilators and 7 requiring BiPAP
- We tested 912 patients for COVID last week in our Urgent Care centers (5% decrease) and the percent positive rate was 28%.
Elective Surgery
Although elective surgeries were suspended as a result of the Covid surge, we continued performing urgent and emergent cases such as cancer and cardiac surgeries during the two-week pause. In evaluating the upcoming weeks, the schedule appears light resulting from physician summer vacations and the approaching holiday weekend. We have also noted that some patients are opting to reschedule elective surgeries to a later date. Taking these factors into consideration along with the decline in Covid cases, we will begin resuming elective surgeries next week. This will assist us in accommodating elective overnight surgeries, which primarily impacts patients requiring orthopedic procedures. We will continue to closely monitor the situation and make adjustments should the trends change.
Therapy
One of the biggest developments during the past week was the Florida announcement to open 21 sites for monoclonal antibody therapy across the state. Each site would be capable of treating more than 300 patients per day. The therapy has received an EUA for individuals 12 years old and above who are COVID positive or who had a significant exposure and who have a risk factor for severe disease (heart disease, lung disease, pregnancy, older age, obesity, immunosuppressive disease, sickle cell disease, and neurodevelopmental disorders). This would allow early treatment of over 6,000 patients per week in these groups.
Vaccination
Vaccination continues to be a primary weapon against the COVID pandemic. The FDA has now given the Pfizer-BioNTech vaccine full approval for ages 16 and older. The vaccine continues to have an EUA for ages 12-15. Pfizer and BioNTech plan to apply for full approval for that age range as soon as the required data is available. Moderna filed for full approval of their vaccine in June, the month following the Pfizer-BioNTech application.
On August 18, HHS Public Health and medical experts issued a joint statement announcing a plan to begin offering booster shots to all Americans on September 20 and starting 8 months following an individual’s second dose of the Pfizer or Moderna vaccine, pending an FDA evaluation of the safety and efficacy of a third dose and the CDC’s AICP (Advisory Committee on Immunization Practices) issuing a recommendation for the booster dose. This followed the recent FDA emergency use authorization for an additional dose of the Moderna or Pfizer vaccine in solid organ transplant recipients or those who have an equivalent level of immunocompromise. The statement did not include the J+J vaccine.
Additional information continues to be released about the effectiveness of vaccines over time, the impact of the variants on the timeline, and the impact of booster doses on immunity. Data from Israel showed that people vaccinated in January with an mRNA vaccine had 2.26x greater risk of breakthrough infection than those vaccinated in April. The data showed that in people 65 years and older who were vaccinated in January, the Pfizer vaccine was less than 55% effective against severe disease and hospitalization. In a study from nursing home residents in the U.S., it was found that the mRNA vaccines had an efficacy against all infections that dropped from 75% pre-Delta to 53% after Delta became the dominant strain. In the UK, protection against symptomatic infection decreased significantly for the Delta period, to 84% for the Pfizer vaccine and to 71% for the AstraZeneca vaccine.
Recently, Israel has experienced a significant surge related to the Delta variant, despite having over 78% of individuals over 12 years old being fully vaccinated. Israel currently has one of the world’s highest infection rates at 650 new cases daily per million people. 59% of individuals hospitalized with severe or critical disease were fully vaccinated. Of the vaccinated, 87% were 60 or older. Based on this data, Israel was one of the first countries to authorize booster doses. They found that booster doses significantly increased protection one week after administration in a study involving 149,000 subjects. The booster decreased the risk of infection by 86% and the risk of severe infection by 92%. This Israeli data was a factor in the recent announcement by HHS related to boosters in all Americans who had received an mRNA vaccine.
The exact type of booster shot is still under investigation. The current plan is to use the same Pfizer or Moderna vaccine for the booster. Moderna and Pfizer are also working on boosters which are specifically tailored to be more effective against the variant strains and the initial data appears promising. In addition, mix and match of vaccine types for booster may be a desirable strategy. In this strategy, a different type of vaccine is given as the booster dose. Early studies have demonstrated even higher levels of antibodies in individuals receiving these boosters. Additional data and details from ongoing studies should be available in September.
We are cautiously optimistic that Florida and our community appear to be past the peak of the Delta variant Covid surge, and the situation will continue to improve over the next few weeks.
CEO Update 8/16/21
The Delta variant driven COVID surge continues to result in increased new cases and hospitalizations both regionally and nationally. Given the increased number of weekly new cases, it is very likely that the strain on hospital systems will continue for the upcoming weeks. The decrease in the (Rt) reproduction time in Florida is cause for optimism, as it suggests that the surge in Florida should moderate in the next few weeks.
Statewide Metrics
- There were 151,415 new cases during the week ending 8/13 (12% Increase)
- The percent positive rate was 19.3% for the week ending 8/13 (Stable)
- The Rt value is 0.99 (covidestim.org 8/13)
- 12.4 million individuals have received at least one vaccine dose (65% of population)
Palm Beach County Metrics
- There were 9,159 new cases during the week ending 8/13 (20% Increase)
- The percent positive rate was 17.8% for the week ending 8/13
- 888,767 individuals have received at least one vaccine dose (68% of population over 12)
Jupiter Medical Center
- There are 79 COVID patients currently hospitalized (25% increase)
- 15 patients are in the ICU with 8 requiring ventilators and 6 requiring Bi-PAP
- We tested 1,065 patients for COVID last week in our Urgent Care centers (27% increase) and the percent positive rate was 27%.
Delta Variant and Current Surge
Nationally, 97.4% of new Covid cases are Delta variants (based on genomic analysis). As detailed above, new cases, hospitalizations, and deaths continue to rise on the state and local levels. Hospitalizations have increased 20% week over week. The Florida Hospital Association reported that 87% of hospitalized patients are unvaccinated, a drop from the early reports that 95% of hospitalizations were in the unvaccinated population. There has also been an increase in pediatric cases and hospitalizations in the 0-17 years-old age groups.
Cancellation of Elective Procedures
We anticipate these high Covid related volumes to continue for the next 1-2 weeks. In order to free up clinical resources, last week we made the decision to cancel and reschedule inpatient elective surgeries that are not considered urgent or emergent, effective Monday 8/16 through Friday 8/20. We will reassess the situation on a regular basis to determine if this will need to be extended, based on bed availability. These steps will assist us in accommodating emergency cases and necessary surgeries that cannot or should not be delayed, while providing needed resources for all of our patients.
The cancellations apply only to inpatient elective procedures that are not considered urgent or emergent. Outpatient elective procedures are not affected at this time.
Update on COVID therapy
In contrast to the great success in vaccine development, finding or developing additional therapeutic agents has been challenging. The mainstay of treatment is dexamethasone, a steroid, which has been shown to reduce the risk of death when used in COVID patients who require oxygen. Remdesivir is also widely used in COVID patients. An early study demonstrated faster recovery time in COVID patients who require low levels of oxygen therapy. In addition, remdesivir did appear to confer a significant survival benefit in this population at 29 days. More recently, baricitinib and tocilizumab may be beneficial in patients who are demonstrating rapidly increasing oxygen requirements.
Monoclonal antibody therapy with casirivimab plus imdevimab or the single agent, sotrovimab have been shown to reduce the risk of hospitalization or death in patients with mild to moderate COVID disease and risk factors for disease progression. Monoclonal antibody therapy has been shown to reduce the risk of severe illness, hospitalization, and death by 70%. These agents have received emergency use authorization from the FDA. In Florida, Governor DeSantis announced a plan to launch rapid response units to offer monoclonal antibody therapy. In addition, strike teams would be deployed to long-term care facilities to administer monoclonal antibody therapy to seniors for which the therapy is indicated.
The World Health Organization has announced that they are relaunching the Solidarity trial to study COVID therapy. Three drugs have been chosen as the initial agents for investigation by an expert panel based on their potential in reducing mortality. Artesunate is a malaria drug with anti-inflammatory properties. Imatinib is a cancer agent that reverses pulmonary capillary leak (a trial in the Netherlands has generated positive data). Inflixamib, a TNF alpha inhibitor with anti-inflammatory properties which is used in rheumatoid arthritis and Crohn’s disease patients.
Currently, there are over 630 drug development programs in planning stages and over 460 trials that have been reviewed by the FDA. 11 therapies have received EUAs and one treatment is currently approved by the FDA for use in COVID-19 (Remdesivir).
Due to the increasing strain on hospitals and health systems across Florida, Covid-19 testing and antibody therapy facilities are being planned and mobilized by state and local authorities.
COVID Prevention
Vaccinations remain essential in our fight to prevent the spread of Covid. Many still have questions regarding vaccination, including those previously afflicted by Covid or those with concerns regarding the effect of Covid vaccines on fertility or pregnancy. Dr. Charles Murphy (JMC’s Chief Quality and Patient Safety Officer) recently convened a panel of medical experts to address these and other vaccine related topics: https://www.youtube.com/watch?v=BjcKAQtetso.
COVID Vaccine Booster
As predicted in our last update, the FDA and CDC are now recommending COVID booster doses for solid organ transplant recipients or individuals with an equivalent level of immunocompromise. Immunocompromised individuals have a higher risk of developing severe disease if they do have a COVID infection. The CDC has concluded that an additional dose of vaccine would increase protection in this vulnerable population. Internationally, Israel has extended their approval of booster to include healthcare workers and individuals over 50 years of age.
CEO Update 8/12/21
Florida is experiencing an unprecedented surge in COVID hospitalizations. Health systems across the state are approaching maximum capacity.
Statewide Metrics
- There were 134,506 new cases during the week ending 8/6
- The percent positive rate was 18.9% for the week ending 8/6
- The Rt value is 1.2
- 12.1 million individuals have received at least one vaccine dose
Palm Beach County Metrics
- There were 7,787 new cases during the week ending 8/6
- The percent positive rate was 17.1% for the week ending 8/6
- 900,000 individuals have received at least one vaccine dose (60% to total population)
Jupiter Medical Center
- There are 63 COVID patients currently hospitalized
- 9 patients are in the ICU with 5 requiring ventilators and 3 requiring Bi-PAP
- We tested 1,065 patients for COVID this week in our Urgent Care centers (27% increase) and the percent positivity rate was 27%
Delta Variant
There continues to be a large surge in COVID cases and hospitalizations triggered by the very contagious Delta variant. New cases in Florida have increased from 45,000 per week to over 134,000 per week over the past month. The reproduction number (Rt) has increased to the 1.3 range during the same time period. Over 90% of the new cases are caused by the Delta variant. Hospitalizations in Florida have also increased dramatically and are currently in the 15,000 range. This is 150% of the peak in July, 2020. Approximately 50% of patients in ICUs in Florida are there with COVID.
Over the past few days, there has been some reduction in the reproduction number (Rt) as shown in Figure 1.
Fig 1. Florida Reproduction Number
While this is certainly good news, there is a lag between the number of new cases and the number of new hospitalizations. The decrease in Rt is encouraging and suggests that we may see an inverted V-shaped spike with a rapid downslope as was seen in England with the Delta variant.
Cancellation of Elective Procedures
We are currently experiencing unprecedented volumes at JMC with 259 patients in house of which 63 are Covid positive patients. These volumes have significantly challenged us from a capacity standpoint. The team is working collectively to expedite discharges to free up beds and accommodate patients from the Emergency Department.
We anticipate these high volumes to continue for the next 1-2 weeks. In order to free up clinical resources, we have made the decision to cancel and reschedule elective surgeries that are not considered urgent or emergent, effective Monday 8/16 through Friday 8/20. We will reassess the situation on a regular basis to determine if this will need to be extended, based on bed availability. These steps will assist us in accommodating emergency cases and necessary surgeries that cannot or should not be delayed, while providing needed resources for all of our patients.
Booster Doses
The need for booster doses of the COVID vaccine and the timing of those boosters is under active study. It is known that the antibody response to vaccination degrades over time. As antibody levels decrease, the individual becomes susceptible to re-infection. The mRNA vaccines are demonstrating effectiveness at the 6-month mark in the 90% range for preventing severe illness or death from COVID infection, including illness caused by the variants. Their effectiveness in preventing infection altogether appears to be significantly less, but the illness is much less severe than in unvaccinated individuals. The FDA is expected to announce a decision to recommend booster doses for the immunocompromised in the next few days.
Thank you for your continued support as we face the next set of challenges posed by the pandemic.
Update 8/12/21
We are offering the Moderna Covid 19 Vaccine to those age 18 and older at our Jupiter Medical Center Urgent Care locations in Jupiter, 5430 Military Trail, Suite 64 and 1335 W. Indiantown Road. Walk-ins welcome, no appointment necessary.
Update 8/2/21
The Delta variant continues to be responsible for the surge in global COVID cases. In the US, Florida accounts for approximately 20% of new cases. On Saturday, Florida set a record for new cases (21,683) and on Sunday, Florida set a record for current hospitalizations (10,207). The Florida data shows that 96% of confirmed hospitalized patients are unvaccinated and 25% of these patients are in the ICU. 14% of hospitalized patients require ventilators.
Florida Metrics
- There were 110,477 new cases during the week ending 7/30 (increasing).
- The percent positive rate averaged 18.1 % for the week ending 7/30 (increasing).
- The retransmission rate (Rt) is 1.3 (increasing).
- 11.75 million individuals (61%) have received at least one vaccine dose.
Palm Beach County Metrics
- There were 5,948 new cases for the week ending 7/30 (increasing).
- The positivity rate was 15.5% for the week ending 7/15 (increasing).
- 848,268 individuals (65%) have received at least one dose of a vaccine.
Jupiter Medical Center Metrics
- There are 46 patients currently hospitalized at JMC with COVID-19.
- 10 patients are in the ICU, with 2 requiring ventilators and 5 requiring BiPAP.
- We tested 449 patients for COVID last week in our urgent care centers and the positivity rate was 31%.
Delta Variant
The delta variant is responsible for a continuing global surge in the COVID pandemic. A significant report in the CDC Morbidity and Mortality Weekly Report came out this week regarding outbreaks including breakthrough infections associated with large gatherings last month in Massachusetts. There were reports of dense gatherings in bars, restaurants, guest houses and rental homes. 469 cases were documented, including 246 (74%) in fully vaccinated individuals. 90% of the infections that underwent genetic sequencing were Delta. 274 (79%) of the vaccinated cases were symptomatic. 5 patients required hospitalization of which 4 were vaccinated. There were no deaths. Among vaccinated people who were infected, the median time from the 2-week mark after full vaccination until the onset of symptoms was only 86 days. Similar cycle times on PCR testing suggests that the viral loads in vaccinated and unvaccinated people were similar. This report is very significant due to the finding of a significant number of breakthrough cases and similar viral loads in vaccinated individuals. The good news is that vaccination was protective in terms of disease severity with only 5 hospitalizations and no deaths.
The delta variant is believed to be as transmissible as chickenpox (Fig 1), with only measles being more transmissible. Viral loads in delta patients have been found to be 1000 times higher than in original COVID infections. Delta infections may also be more severe, with a Scottish study demonstrating that the risk of hospital admission was doubled. Similar findings were reported in Canada and Singapore. In the US, rates of hospitalization in the age 18-49 age group have increased by 40%. In England, people younger than 50 were 2.5 times more likely to be infected than older people (prevalence, 0.20% vs 0.08%).
Given this new information, the CDC revised their guidelines related to mask wearing. This was based on the new data showing similar viral loads in vaccinated patients and the knowledge that these individuals can be asymptomatic. In addition, even vaccinated individuals are at risk of COVID infections from the new variants in areas with high levels of transmission. The CDC is therefore recommending that fully vaccinated individuals wear masks in public indoor settings in areas with substantial or high transmission or if they are immunocompromised or at increased risk for severe disease.
COVID Vaccine Booster Doses
Vaccines continue to demonstrate very good protection from COVID infection caused by the variants. Three studies from Canada, Singapore, and Scotland demonstrated that the Pfizer vaccine was more than 90% effective against hospitalization or death from the Delta variant. However, in Israel, 60% of hospitalized patients had been fully vaccinated. In England, 23% of hospitalized patients had been vaccinated. Israel is now giving booster doses to individuals over 60 years of age in addition to those who are immunocompromised. England and Germany have also announced plans to offer vaccine booster shots this fall.
While it is known that the Pfizer and Moderna mRNA vaccines provide the most robust immune response, it is uncertain how long the protection will last. Recent modelling studies have predicted that 50% efficacy will be reached in about 250 days following the mRNA vaccines (Fig 2). Obviously, last week’s data showing a median interval of only 86 days from vaccination to infection is concerning, although, there were no deaths reported in this group. Pfizer has already applied for an EUA (Emergency Use Authorization) for a booster dose of their vaccine. Recently, 2 San Francisco hospitals reported that over 200 of their staff members had tested positive for COVID. About 80% of these individuals had been vaccinated and most of the cases were breakthrough infections related to the Delta variant.
We anticipate that a recommendation will be forthcoming for booster doses in the upcoming weeks by the relevant US agencies which will be evidence-based and stratified by the risk of COVID infection in various populations.
Our team continues to provide care to an increasing number of COVID patients. We are seeing an uptake in critically ill patients, even in younger age groups. We continue to recommend vaccination for all individuals who qualify for the vaccines. This remains the single best way to prevent severe COVID infection or death.
Update 7/20/21
The emergence of the Delta variant is fueling the resurgence of COVID cases globally.
Statewide Metrics
- There were 45,604 new cases during the week ending 7/15 (increasing).
- The percent positive rate averaged 11.5 % for the week ending 7/15 (increasing).
- The retransmission rate (Rt) is 1.2 (increasing).
- 11.2 million individuals (59%) have received at least one vaccine dose.
Palm Beach County Metrics
- There were 2,483 new cases for the week ending 7/15 (increasing).
- The positivity rate was 9.6% for the week ending 7/15 (increasing).
- 820,849 individuals (63%) have received at least one dose of a vaccine.
Jupiter Medical Center Metrics
- There are 28 patients currently hospitalized at JMC with COVID-19.
- 1 patient is in the ICU and is requiring BiPAP.
- We tested 313 patients for COVID this week in our urgent care centers. The positivity rate was 23%.
Delta variant
The Delta variant, also known as the B.1.617.2 variant, is the dominant strain in the US and accounts for 58% of new infections. It is present in all 50 states and new case rates are increasing in most states. The Delta variant appears to be 50% more transmissible than the Alpha (UK) variant, which was itself 50% more transmissible than the original variant. Based on data from the UK, the Delta variant may be more likely to cause hospitalization and death. Vaccination may be slightly less effective against the Delta variant. Two doses of the Moderna or Pfizer vaccine are 88% effective against Delta variant infection and 96% effective against hospitalization. The AstraZeneca vaccine is 60% effective against infection and 93% effective against hospitalization or death. In the US, new cases, new hospitalizations, and percent positivity are all increasing at the present time. National forecasts are predicting an increase in the number of new hospital admissions over the next 4 weeks. Models are also predicting an increase in Florida.
The vast majority of the new COVID infections are in unvaccinated people. Only 6,000 cases of breakthrough infections have been reported, which is 0.003% of those vaccinated. Current hotspots are being seen in Arkansas and Missouri, where only 35% of the population has been fully vaccinated. Hospital resources are strained, with hospitals in the Springfield Missouri area having more than 100 COVID patients each and needing to borrow ventilators to meet demand. While Florida has a higher percentage of its populace that are fully vaccinated (50%), there are still 9.5 million residents who are not fully vaccinated and over 500,000 individuals over 65 who are not vaccinated. Models are predicting a nationwide surge in cases over the next 2 months related to the Delta variant. The number of new cases may be underestimated because vaccinated individuals who contract COVID may be asymptomatic or only exhibit mild symptoms.
As noted above, vaccination is effective against the Delta variant. Some areas are also reinstating other public health measures such as wearing masks indoors regardless of vaccination status (Los Angeles). Some countries have also reinstated restrictions on bars, restaurants, and nightclubs. The World Health Organization has encouraged people to continue to wear masks and practive social distancing. Most of the hospitalizations and deaths are occuring in individuals who are not vaccinated, underscoring the importance of vaccination.
COVID Therapy
Steroids and Remdesivir remain the mainstays of therapy for hospitalized patients with COVID. In addition, baricitinib or toclizumab are indicated in patients with rapidly increasing oxygen requirements. Steroids demonstrate a survival benefit in hospitalized patients requiring supplemental oxygen. Remdesivir demonstrates an improved time to recovery in patients requiring supplemental oxygen and is the only medication which is FDA approved for the treatment of COVID. Convalescent plasma, which contains antibodies against COVID, is also being used in hospitalized patients. Clinical trials are underway to identify which subsets of patients benefit from this therapy.
For outpatients, treatment recommendations have changed as the new variants have emerged. Monoclonal antibody therapy with bamlanivimab with or without etesevimab is no longer recommended because of the new variants. Monoclonal antibody therapy with casirivimab plus imdevimab or with sotrovimab is recommended in outpatients who are at high risk of disease progression. If a COVID patient requiring oxygen is not admitted to the hospital, the NIH panel recommends treatment with dexamethasone for up to 10 days.
Booster Vaccine Doses
As cases counts increase, debate is emerging regarding whether people will need to receive booster shots of the vaccines. On July 8, Pfizer and BioNTech announced that they would be applying for an emergency use authorization (EUA) for a booster dose of their vaccine, citing internal evidence that their vaccine efficacy is waning over time, and a booster dose may be required 6-12 months after vaccination. In response, the FDA and the CDC jointly announced that individuals do not need a booster shot at the present time. While antibody levels do fall over time, this is not the entire story related to immunity. There is data that demonstrates protection from the current vaccines may last for years because B and T cells also participate in the immune response and create germinal centers which contain memory cells. These memory cells can provide long-term immunity. A study published in Nature in June demonstrated that mRNA based vaccination of humans induces a potent germinal center response, which could potentially provide protection for years. Preliminary data out of Israel indicates a decline to 64% in the Pfizer vaccine’s efficacy in preventing infection. However, the vaccine is still 93% effective in preventing hospitalization or death. As a result, Israel has begun administering booster doses to some immunocompromised patients. Additional studies are needed to fully understand the time course of vaccine protection and the need for booster doses of vaccine.
While the ebb of COVID cases during the spring was a welcome development, we have been aware that future surges fueled by variants was a possibility. JMC has continued to maintain our preparedness for COVID testing, treatment and vaccinations.
As mentioned above, the World Health Organization is encouraging people to continue to wear masks and practice social distancing. The current surge of the pandemic is largely afflicting the unvaccinated, so please consider vaccination if you have not already done so.
Update 6/16/21
We have exhausted our supply of vaccine and are no longer scheduling appointments. To keep phone lines open for medical emergencies, please continue to check this page for updates. Thank you for your patience and continued support of Jupiter Medical Center.
Patients who received first dose vaccinations at Jupiter Medical Center will be able to receive their second dose at our facility.
Update 04/14/21
Headlines regarding the COVID pandemic continue to be centered on the presence of significant surges around the globe, the impact of variants on case rates and therapies, and the development of new therapies. In addition, the FDA and CDC have recommended pausing the use of the J+J vaccine in the US due to the occurrence of rare cases of blood clotting associated with low platelet counts.
Statewide Metrics
- There were 5,558 new cases on 4/10 and 41,896 in the week ending 4/4 (increasing).
- There were 3,023 patients hospitalized on 3/29 (increasing).
- The percent positive rate averaged 7.47% for the week ending 4/4 (increasing).
- The retransmission rate (Rt) is 1.1 on 4/10 (stable).
- 7.2 Million individuals have received at least one vaccine dose.
Palm Beach County Metrics
- There were 346 new cases on 4/10 and 2,778 new cases for the week ending 4/4 (increasing).
- The positivity rate was 6.93% for the week ending 4/4 (increasing).
- 528,000 individuals have received at least one dose of a vaccine.
Jupiter Medical Center
- There are 10 patients currently hospitalized at JMC with COVID-19.
- 5 patients are in the ICU (2 requiring ventilators and 3 requiring BiPAP).
- We tested 328 patients this week in our urgent care centers for COVID (9.7% increase).
Global Surges
There continue to be significant surges around the globe. Important factors are believed to be the impact of variants, the easing of social distancing and mask requirements, and the challenges of vaccinating populations in a timely manner. India is setting records of 68,912 new cases per day. Only 1% of their population is vaccinated. Additional surges are being seen in Europe, Brazil, and the Philippines.
There continue to be increasing new case numbers and hospitalizations in the US. 5 states account for 42% of the new cases (Michigan, New York, Florida, Pennsylvania, and New Jersey). Case rates for children under 19 are at record rates. Epidemiologists have identified school sports as a major source of spread.
Variants
The variants continue to play a critical role in the pandemic. They are a major factor in the new surges that have occurred. The CDC has developed a classification system based on the characteristics of the variants and resulting actions and consequences for public health. The 3 categories are variants of interest, variants of concern, and variants of high consequence. There are currently 5 variants of concern and no variants of high consequence in the US.
Israel has published new data that indicates that the South African variant is breaking through the Pfizer vaccine in Israeli study. The study compared over 400 people who tested positive more than 14 days after receiving one or two doses of the vaccine compared to the same number of unvaccinated patients with COVID. They found that the prevalence rate of the variant was 8x higher in the vaccinated versus the unvaccinated group (5.4 vs 0.7%). This suggests that the vaccine is less effective against the South African variant.
Vaccines
The FDA and the CDC have now recommended pausing the use of the J+J vaccine due to the presence of rare cases of blood clotting associated with low platelet counts. There have been 6 cases reported in women between 18 and 48 years of age who had received the vaccine between 6 and 13 days prior to developing the blood clots. These events are similar to the ones seen with the AstraZeneca vaccine. Both of these vaccines use the adenovirus vector as the framework for the vaccine. A possible mechanism for the clotting is that the antibodies stimulated by these vaccines are binding to platelets and causing the body to inappropriately destroy platelets, which are a key element in the normal clotting process. A similar issue is seen in some patients who receive heparin. This story is just coming out and we will be following developments closely.
Pfizer has requested the FDA to expand the emergency use of their COVID vaccine to adolescents aged 12 to 15. Pfizer had reported that the vaccine was found to be safe, effective and produced a robust antibody response in 12 to 15 year olds in a clinical trial involving 2,260 patients. The vaccine was 100% effective in preventing illness in the trial.
China’s top disease control official acknowledged that the currently available Chinese vaccines do not have high protection rates at preventing symptomatic COVID infections. The efficacy rates reported by the companies have ranged from 50-79%. The threshold considered necessary for vaccines is at least 50%. He stated that mixing vaccines is among strategies being considered to boost their effectiveness. In addition, mRNA vaccines developed in China are now entering clinical trials.
Therapy
Regeneron has reported that their antibody therapy Regen-COV reduced the risk of developing symptomatic COVID by 81% compared with placebo in people living with someone infected with COVID. They will ask the FDA to approve its use in people exposed to the virus who have not been vaccinated.
On April 8th, the NIH COVID-19 Guidelines Panel recommended the use of combination anti-SARS-CoV-2 monoclonal antibodies to treat outpatients with mild to moderate COVID-19 who are at high risk of clinical progression. The treatment should be administered as soon as possible after a positive COVID test and within 10 days of symptom onset. The panel recommends against the use of monotherapy with bamlanivimab due to variant resistance.
World’s Best Hospitals 2021
Newsweek has released its World’s Best Hospitals 2021 list (see cover below). Utilizing criteria selected by medical experts from around the world (United States, Germany, France, Switzerland and Israel), the publication partnered with Statista (a global data research firm) to compile this group.
The criteria included:
- Medical KPI’s (key performance indicators)
- Patient surveys
- Recommendations from medical experts (doctors, hospital managers, health care professionals)
Using these criteria, approximately top 2% of the 164,500 hospitals globally and roughly top 5% (world class) of the 6,090 hospitals in the Unites States were identified.
I am proud to announce that Jupiter Medical Center is honored on this list and is the only hospital representing Palm Beach County and the Treasure Coast.
According to Newsweek, the World’s Best Hospitals stand out for their consistent excellence, including distinguished physicians, top notch nursing care and state-of-the-art technology.
Given the enormity of the obstacles presented by the Covid-19 pandemic and the challenges inherent in launching new programs (cardiac surgery, medical oncology, women’s & children’s), this is an incredible achievement by our medical staff and the entire Jupiter Medical Center team.
Update 03/30/21
A Message from the CEO Amit Rastogi, MD, MHCM
The critical elements of the Covid pandemic are the occurrence of significant new global surges, the proliferation of variants and the race to vaccinate populations as a mitigation strategy.
Florida Metrics
- There were 4,943 new cases on 3/28 and 35,551 in the week ending 3/21 (increasing).
- There were 2,871 patients hospitalized on 3/29 (stable).
- The percent positive rate averaged 6.62% for the week ending 3/21 (increasing).
- The retransmission rate (Rt) is 1.1 on 3/28 (increasing).
- 5.6 million individuals have received at least one vaccine dose.
Palm Beach County Metrics
- There were 369 new cases on 3/28 and 2,551 new cases for the week ending 3/21 (increasing).
- The positivity rate was 6.6% for the week ending 3/21 (increasing).
- 415,000 individuals have received at least one dose of a vaccine.
Jupiter Medical Center Metrics
- There are 14 patients currently hospitalized at JMC with Covid-19.
- 2 patients are in the ICU (both requiring BiPAP).
- We tested 316 patients this week in our urgent care centers for Covid-19 (17% increase).
COVID Surges
There continue to be significant surges around the world, Europe has been hit hard in the most recent uptick. New case rates have exceeded 1,000 new cases/100,000 population (the current rate in the US is 127.4/100,000). In response, France has imposed a partial lockdown and Italy has announced a full Easter lockdown. Physicians in France have warned that ICUs will likely be overwhelmed and care rationing may need to be implemented. South America has also seen a significant increase in cases and Brazil’s healthcare system is strained with >80% of ICU beds being filled. These trends are important to monitor because prior surges in Europe predated surges in the US by 2-3 weeks.
After a plateau had been reached following the surge in December/January, there has been a recent uptick in the US new case rates by 6.7% week over week (March 24). Florida’s new case rate has also started trending up as has the percent positive rate for testing. In addition, the reproduction rate (Rt) has increased to 1.1 in Florida, which also indicates that new cases are increasing. The contributions of variants versus other factors is not clear and Spring break/Easter gatherings may contribute to an increase in case numbers. It is not believed that vaccinations have reached levels necessary to prevent surges.
Variants
Variants continue to be of concern because of the increased transmissibility of some of the strains, the possibility of variants causing more severe disease, and the potential for allowing vaccine escape. The most recent data from the CDC demonstrates that the B.1.1.7 variant may now account for >20% of Covid cases in the US. The graph below shows the significant increase in this variant of concern since January 1st. This variant has been found to be 50% more transmissible than the baseline virus and some reports have demonstrated a higher mortality rate from this variant (which was first identified in Great Britain). As of February 27th, 13.2% of Covid cases in Florida were identified as B.1.1.7.
An important recent finding has been the significantly impaired effectiveness of single agent monoclonal antibody therapy against the Covid variants. A recent study demonstrated that bamlanivimab was not effective in vitro against viruses containing the mutations of the B.1.1.7 (UK) or the B.1.351 (South African) variants. Based on this information, the US has halted the distribution of the single monoclonal antibody agent. It appears that combination monoclonal antibody therapies continue to demonstrate efficacy against the variant strains and the use of these products in outpatients with mild to moderate disease should still be considered. An additional disappointing finding is the decreased effectiveness of convalescent plasma against the variant strains, particularly against the South African variant.
Vaccinations
In an effort to mitigate the effects of Covid-19 surges and variants, significant progress continues in vaccinating our citizens. Nationally, 2.5 million vaccines are being administered each day. In Florida, 25.8% of the population has received at least one dose. As of Monday March 29th, persons 40 years of age and older are eligible for vaccines and as of April 5, persons 18 years of age and over will be able to be vaccinated in Florida. Jupiter Medical Center continues to provide vaccinations to our community in line with the state guidelines. Our primary focus in recent months has been providing vaccines to individuals with comorbidities that make severe disease more likely. We are now expanding our recipient pool based on state and federal guidelines. We are proud that we have been able to provide this critical service to our community.
Doctor’s Day
On behalf of all JMC team members and our boards, I would like to thank our entire medical staff for their tireless efforts during the pandemic. The dedication and commitment of our physicians in collaborating with us to develop Covid safety/treatment protocols, vaccine prioritization and most importantly, selflessly taking care of our sickest patients, proved to be an invaluable asset to our community when we needed them the most.
Happy Doctor’s Day!
Visiting Hours Updates
Effective March 15, 2021
The health and safety of our patients, visitors, team members and community is our top priority, our visitor policy is as follows:
- Inpatients will be allowed one designated visitor from 1pm - 5pm. and will enter through the East Entrance. Upon arrival, visitors will be screened, provide identification, are required to wear a mask and will be directed to the patients room where they will need to remain during their visit.
- Labor & Delivery and Pediatric patients may have one visitor accompany them during their stay.
- Parents with an infant in the neonatal ICU will be allowed to be with their infant, one parent at a time.
- Other exceptions, including visits to hospice, will be considered on a case-by-case basis.
Visitors who are sick are not permitted to enter the hospital, without exception. Visitors will be screened at the time of arrival.
Hospital Entrance
All visitors are now being directed to enter the hospital through the East Entrance.
Update 03/2/21
A Message from the CEO Amit Rastogi, MD, MHCM
News concerning vaccines continues to be the dominant theme concerning the Covid pandemic and there are numerous developments.
- A third vaccine developed by Johnson and Johnson (J&J) has been approved in the US and shipments have begun this week.
- Pfizer was granted permission by the FDA to store their vaccine in regular freezers for up to 2 weeks.
- Trials are currently underway involving individuals in the 12-18 age range. It is believed that the emergency use authorization may be extended to this group before the next school year begins.
- Vaccination for younger children may require full evaluation by the FDA rather than an emergency use authorization, due to the lower risk of Covid in these individuals.
- In Florida, Governor DeSantis announced that police, firefighters, and teachers over 50 years of age are now able to receive vaccinations at four FEMA sites in Florida.
Statewide Metrics
- There were 1,817 new cases on 2/28 and 40,000 in the week ending 2/21 (decreasing).
- There were 3,687 patients hospitalized on 3/2 (decreasing).
- The percent positive rate averaged 6.39% for the week ending 2/21 (decreasing).
- The retransmission rate (Rt) is 0.92 on 2/28 (stable).
- 3 million individuals have received at least one vaccine dose.
Palm Beach County Metrics
- There were 412 new cases on 2/27 and 3,140 new cases for the week ending 2/21 (decreasing).
- The positivity rate was 6.67% for the week ending 2/21 (decreasing).
- There was a decrease in Covid-like illness cases to 435 for the week ending 2/21 (decreasing).
- 265,000 individuals have received at least one dose of a vaccine.
Jupiter Medical Center
- There are 13 patients currently hospitalized at JMC with Covid-19.
- 2 patients are in the ICU (1 requiring ventilator support and 1 requiring BiPAP support).
- We tested over 400 patients this week in our urgent care centers for Covid.
Covid-19 vaccines and variants
The J&J Covid vaccine received Emergency Use Authorization (EUA) from the FDA on February 28th after the FDA advisory panel unanimously recommended that action on Friday. The CDC director approved the vaccine on Sunday and distribution of up to 4 million doses of the vaccine has begun this week. The J&J vaccine is based on viral vector technology. A common cold virus called adenovirus 26 is genetically engineered to cause human cells to manufacture a fragment of the Covid spike protein. This causes the body to develop an immune response to the spike protein and thus Covid. The altered adenovirus does not cause recipients to develop a cold. Advantages of this vaccine are that it only requires one shot and it can be kept in regular refrigerators for up to 3 months. It is also significantly less expensive than the Pfizer or Moderna vaccines. Protection from the J&J vaccine begins about two weeks after the shot; zero hospitalizations or deaths were seen by four weeks after the dose.
The EUA request was based on a Phase 3 trial involving 44 thousand individuals. The vaccine is highly effective in the prevention of severe/critical Covid disease. The vaccine prevented all hospitalization and deaths 28 days after vaccination and is highly effective against the South African variant. The vaccine also has an excellent safety profile. Based on this data, individuals should be vaccinated with any of the three vaccines that have received emergency use authorization, J&J, Moderna or Pfizer. All three are highly effective at preventing severe Covid disease and its complications.
As vaccine production accelerates, the important topic of the vaccination of children against Covid is coming to the forefront. Moderna and Pfizer began enrolling children 12 years of age and older in clinical trials and plan to have results by the summer. The FDA will then review the results of these trials before deciding to extend the emergency use authorizations to this population. Only 1% of Covid deaths occur in individuals under 21 years of age. However, about 2% of children who develop Covid require hospitalization and 227 children have died of Covid. Moving below 12 years of age will likely require new studies and modifications of the doses due to an enhanced response in children. It is likely that the FDA will perform a complete analysis related to children under 12 years of age and that the approval would not occur in the form of an EUA.
In Florida, Governor DeSantis announced that police, firefighters, and teachers over the age of 50 will be able to receive vaccinations at the 4 FEMA centers located in Miami, Jacksonville, Orlando, and Tampa. He also extended the vaccine program for at risk individuals under the age of 65 with comorbidities to physicians, advanced practice providers, and pharmacies. This will require availability of vaccine doses at these sites before operationalization and a state form completed by a provider.
Jupiter Medical Center continues to be active in both the prevention and treatment of Covid disease. We are continuing with the very successful program to vaccinate individuals under 65 years of age with comorbidities in partnership with community physicians. We anticipate continuing to provide this important service to our community based on vaccine allocations from the state. In addition, we are one of the few hospitals in the region that continues to provide monoclonal antibody therapy early in Covid disease to prevent hospitalizations and severe disease. Our teams continue to provide outstanding care to patients with moderate and severe Covid disease who require hospitalization. We are very proud of the sustained response to the pandemic by all members of the JMC family.
Update 02/25/21
Jupiter Medical Center received a limited supply of COVID 19 vaccine. The focus was on patients age 18-64, residing in Palm Beach or Martin Counties, and have comorbidities. We have completely exhausted our supply of vaccine. If you were not contacted with an appointment time, we reached capacity and are no longer scheduling appointments.
To keep phone lines open for medical emergencies, please continue to check this page for updates. Thank you for your patience and continued support of Jupiter Medical Center.
Update 02/17/21
A Message from the CEO Amit Rastogi, MD, MHCM
The dominant themes concerning the COVID-19 pandemic are the significant reduction in cases and hospitalizations from the peak levels of the recent surge, the progress towards vaccination of the population, and the impact of COVID-19 variants. In the U.S., vaccine supply constraints are beginning to ease as production accelerates and vaccination channels proliferate. Over 10% of the US population (37 million people) has received at least one dose of a COVID-19 vaccine. In Florida, 2.3 million individuals have received at least one dose (10% of the population). Unfortunately, Florida also has the most confirmed cases of the U.K. variant, with 347 cases reported as of February 11. We are watching the data very closely as the U.K. variant is expected to be the dominant strain in the U.S. by March. It is certainly possible that another surge could be the result and it is essential that we continue to protect ourselves and our community.
Statewide Metrics
- There were 3,615 new cases on 2/15 (decreasing).
- There were 4,689 patients hospitalized on 2/14 (decreasing).
- The percent positive rate averaged 7.25% for the week ending 2/7 (decreasing).
- The retransmission rate (Rt) is 0.91 on 2/14 (stable).
Palm Beach County Metrics
- There were 333 new cases on 2/15 and 3,382 new cases for the week ending 2/7 (decreasing).
- The positivity rate was 7.32% for the week ending 2/7 (decreasing).
- There was a decrease in COVID-like illness cases to 502 for the week ending 1/31 (decreasing).
Jupiter Medical Center
- There are 15 patients currently hospitalized at JMC with COVID-19.
- 3 patients are in the ICU (1 requiring BIPAP).
- We tested over 400 patients this week in our urgent care centers for COVID-19.
Covid-19 vaccines and variants
Early data from COVID-19 vaccinations is giving hope to people across the globe. Some of the most compelling data is coming from Israel, which has vaccinated over half of eligible residents (3.5 million people) with 69.4 doses per 100 people. In contrast, the rate in the U.S. is 15.4 doses per 100 people. Over 90% of individuals over age 60 have been vaccinated in Israel, resulting in a 40% drop in confirmed new cases and a 30% drop in hospitalizations. This is compared to a 12% drop in new cases and a 5% drop in hospitalizations in a younger age group, in which only 30% have been vaccinated.
There is also data which indicates that vaccinated individuals are far less likely to spread the virus. In Moderna’s phase 3 trial, there was an 89% reduction in asymptomatic and symptomatic cases prior to the second dose of the vaccine.
Clearly, vaccination is an important way to reduce one’s risk related to Covid and many who have been vaccinated are wondering what they can safely do following vaccination. There is evidence that maximum protection occurs about 3 weeks following the second dose of the Pfizer vaccine, which should also be applicable to Moderna.
It continues to be unknown how long immunity from full vaccination will last. It is very possible that a yearly booster will be required. It is also possible that an additional dose of a modified vaccine tailored to the variant strains will also be necessary.
The CDC has advised that people who have received the full course of COVID-19 vaccines can skip the standard 14-day quarantine after exposure to someone with infection, as long as they remain asymptomatic.
While the aforementioned provides significant optimism, there are individual differences in development of immunity following vaccination. Furthermore, the detection of new variants raises concern of vaccine efficacy against these variants. Viruses constantly mutate, but particular combinations of mutations can result in vaccine escape. Influenza, which mutates much faster than coronavirus, requires modification of flu shots every year, resulting in development of a global flu vaccine system. Similar tracking will need to be established for COVID-19 and fortunately, the vaccines are based on a flexible technology that can be updated for booster development as needed.
As a result, vaccinated individuals should continue to wear masks, socially distance, and avoid large gatherings to decrease transmission and the risk of acquiring COVID-19.
The U.S. continues to ramp up the pace of vaccinations. Experts have stated that vaccinations could become widely available by May. The government has begun shipping vaccine to pharmacies. In phase one, one million doses were sent to 6,500 pharmacies. 21 pharmacy networks including over 40,000 pharmacies are participating in the program. CVS, Walgreens, Walmart, and Publix pharmacies are included in the program. Many pharmacies already provide vaccinations for illnesses such as the flu and shingles. It is believed that the pharmacies could administer up to 100 million doses of COVID-19 vaccine a month. It is estimated that over 70% of the U.S. population could have received at least one dose by mid-September.
In Florida, pharmacies are focusing on vaccinating those over age 65 while some hospitals are being asked to partner with state organizations to focus on providing vaccination to individuals under age 65 with serious comorbidities. Jupiter Medical Center is proud to be one of the hospitals serving this group. In the first phase of this initiative, we have partnered with hundreds of community physicians to vaccinate at-risk individuals that they identify as most vulnerable. Later this week, JMC will be launching the next phase, which will provide direct community outreach to this population via our website. Details will be posted on our website in the upcoming days.
These developments continue to offer hope for a return to a more normal state over the next 6-9 months. Continued vigilance and compliance with masks, social distancing, and avoiding large groups remains important in the fight against the pandemic.
As always, thank you for your continued support of Jupiter Medical Center.
Update 02/03/21
COVID-19 cases in the U.S. and Florida continue their downward trend. In Florida, new cases, percent positivity, and hospitalizations have all been decreasing over the past 3 weeks. We expect this trend to continue as vaccination efforts accelerate.
Statewide Metrics
- There were 70,727 new cases during the week ending 1/24 (decreasing).
- There were 6,109 patients hospitalized on 2/2 (decreasing).
- The percent positive rate averaged 9.26% for the week ending 1/24 (decreasing).
- The retransmission rate (Rt) is 0.91 on 2/2 (decreasing).
Palm Beach County Metrics
- There were 349 new cases on 2/1 and 4,899 new cases for the week ending 1/24 (stable).
- The positivity rate was 9.09% for the week ending 1/24 (stable).
- There was a decrease in COVID-like illness cases to 661 for the week ending 1/24 (decreasing).
Jupiter Medical Center
- There are 23 patients currently hospitalized at JMC with COVID-19.
- 4 patients are in the ICU (1 requiring ventilator support and 1 requiring BIPAP support).
- We tested over 500 patients last week in our urgent care centers for COVID-19, a 50% decrease since last month
Covid-19 vaccines
In the U.S., 26 million people (7.8% of the population) have received one or more doses of the COVID-19 vaccine. In Florida, over 1.7 million individuals (7.7%) have received at least one dose. Vaccine supply continues to be the rate limiting step, prompting President Biden to order a 16% increase in shipments to the states.
As a result, Florida received 307,000 doses of vaccine last week instead of the prior 266,000 weekly doses. A portion of the increased supply this week has been allocated towards medically vulnerable individuals between the ages of 16 and 64 years old and JMC is very pleased to be one of the hospitals which will participate in serving this population. This population was mentioned in Governor Desantis’ executive order regarding priority populations and was included in the 1C group of the initial CDC Advisory Committee for Immunization Practices Guidelines for vaccine allocation. Certain medical conditions which include COPD, chronic kidney disease, diabetes, and heart failure are associated with more severe COVID-19 illness. We are partnering with medical staff primary care physicians in the community to identify individuals to vaccinate in this high-risk population.
The over age 65 population continues to be a priority and mass vaccination site(s) with the capacity to administer thousands of doses daily are being planned in Palm Beach County. Publix pharmacies continue to serve as a vaccine source for this population. As many of you are aware, Jupiter Medical Center has a Publix pharmacy partnership on our campus. After discussions with Publix leadership, we are pleased to announce that the Publix pharmacy on JMC campus has been added as a vaccination site for those over age 65. Appointments for this site can only be made via the Publix website.
While vaccine demand still far outpaces supply, we are encouraged by the increase in shipments to the states. Furthermore, pending FDA approval, the J&J vaccine will provide an additional boost to supply by March/April.
Rest assured that the JMC team will continue to advocate to serve as a vaccine resource for our community to provide as many doses as possible, as quickly as possible.
Thank you for your patience as we continue our fight against the pandemic and thank you for your continued support of Jupiter Medical Center.
Sincerely,
Amit Rastogi, MD, MHCM
Update 02/02/21
Jupiter Medical Center has received a very limited supply of the Moderna vaccine from the State. This allotment is focused on patients who are age 18-64 and have co-morbidities which increase the severity of illness from Covid-19. The CDC has identified comorbidities which are known to increase the chance of severe illness and include:
- Cancer (active, not history of)
- Chronic kidney disease
- COPD
- Heart conditions (CHF, cardiomyopathies, CAD)
- Solid organ transplantation
- Obesity (BMI ≥ 30 kg/m2)
- Sickle cell disease
- Down’s Syndrome
- Diabetes
We are partnering with medical staff primary care physicians in the community
to identify individuals to vaccinate in the high-risk population. Patients
who meet criteria will be submitted through their PCP and then scheduled
to have the vaccine administered.
We will not be booking appointments through our website. We had hoped to receive more doses of vaccine that would allow us to vaccinate
more of these patients at increased risk, but our supply is very limited.
To keep phone lines open for medical emergencies, please continue to check this page for future updates. Your continued support of Jupiter Medical Center is greatly appreciated.
Updated 01/29/21
While numerous countries across the globe continue to grapple with the spread of COVID-19, new cases and related hospitalizations in the US are finally decreasing. Due to the lingering effects of post-holiday travel and the discovery of new variants, we must approach this news with cautious optimism.
Statewide Metrics
- There were 76,697 new cases during the week ending 1/17 (decreasing).
- There were 6910 patients hospitalized on 1/25 (decreasing).
- The percent positive rate averaged 10.05% for the week ending 1/17 (decreasing).
- The retransmission rate (Rt) is 0.96 on 1/25 (stable).
Palm Beach County Metrics
- There were 592 new cases on 1/24 and 4,783 new cases for the week ending 1/17 (decreasing).
- The positivity rate was 9.27% for the week ending 1/17 (stable).
- There was a decrease in COVID-like illness cases to 632 for the week ending 1/10 (decreasing).
Jupiter Medical Center
- There are 33 patients currently hospitalized at JMC with COVID-19.
- 6 patients are in the ICU (2 requiring ventilators and 1 requiring BIPAP support).
- We tested over 600 patients per week in our urgent care centers for COVID.
Covid-19 variants and vaccines
Multiple variants of the COVID-19 virus have been identified. Current investigations are underway to define the transmissibility, severity of illness, and the susceptibility to current vaccines of these variants. Recent data has demonstrated that the UK variant is 30-70% more transmissible than the original and suggests that it is more lethal.
President Biden has issued several executive actions aimed at improving the national pandemic response. In an effort to stem the tide of new COVID-19 variants entering the US, a ban is being reinstated on many non-US citizens attempting travel to the US. Additionally, the CDC has announced that it will require all travelers flying to the US from abroad to show proof of a negative coronavirus test before getting on a plane.
Vaccine development, delivery and administration are central to any COVID-19 mitigation strategy and numerous pharmaceutical companies have been pursuing vaccine development. The maker of the single dose vaccine, J&J, is expected to report critical data in approximately 2 weeks, which will set the stage for the FDA issuing an EUA (Emergency Use Authorization) in late February or early March for the vaccine. Although the Astra Zeneca vaccine has been approved for use in Europe, a significant issue with the trial used for approval in Europe has caused the company not to submit for a United States EUA and the FDA will perform an independent analysis. Merck reported disappointing results with both of their candidate vaccines and has subsequently stopped clinical trials.
One of the major issues related to vaccines has been supply. Both Moderna and Pfizer committed to deliver 100 million doses each by the end of March. So far, they have delivered 36 million doses and are currently delivering 12-18 million doses per week. In order to boost supply, President Biden said his administration will soon be able to confirm the purchase of an additional 100 million doses each of the Pfizer and Moderna vaccines, with the shipments to be delivered by this summer. The new order represents a 50% increase in the country’s total vaccine supply from 400 million ordered doses to 600 million.
Until the aforementioned steps take effect, it is unlikely that Florida’s vaccine shipments from the federal government will surpass the current rate of approximately 250,000 doses per week. In an effort to rapidly increase vaccination sites, state authorities are directing vaccine supply towards Publix pharmacy sites, now expanded to over 100 pharmacies in 12 Florida counties. As a result, it is doubtful that hospitals and perhaps even county departments of health will receive additional vaccine going forward.
For those of you that received your first vaccine dose (either Moderna or Pfizer) at Jupiter Medical Center, I am pleased to announce that we have received second dose shipments for both.
As COVID-19 cases begin to ebb and vaccine administration accelerates, we appear to be gaining momentum in the battle against the pandemic. Let’s all continue to do our part by practicing social distancing and wearing masks.
Thank you for your continued support of Jupiter Medical Center.
Updated: 01/22/21
Nationally, the number of new COVID-19 cases has now declined from the most recent peak. There continue to be significant hotspots in COVID-19 activity across the U.S. following the holidays. California and Arizona have seen significant surges in the number of new cases. In Arizona, the daily death rate has increased from 90 to 160 deaths over the past 2 weeks. Review of recent data in Florida suggests that we may be starting to see some moderation after the most recent surge. The Rt currently stands at 0.93, below the important threshold of 1.0. Number of new cases and percent positivity rates are both decreasing. There has also been a trend of decreasing COVID-like illness presenting to emergency rooms over the past week.
The impact of the variant COVID-19 viruses, which appear to be more transmissible, remains uncertain. The United Kingdom has seen a significant acceleration in new case rates which is attributed to the variants. It is predicted that the U.K. variant may become the dominant virus in the U.S. by March.
Efforts to vaccinate key populations have been accelerating. Over 12 million people have received initial vaccine doses nationally, which is about 3.6% of the population. In Florida, almost one million individuals (4.5%) have received their first shot.
Statewide Metrics
- There were 92,485 new cases during the week ending January 10th (decreasing).
- There were 7420 patients hospitalized on January 18th (decreasing).
- The percent positive rate averaged 10.73 for the week ending January 10th (decreasing).
- The retransmission rate (Rt) is 0.93 on January 19th (decreasing).
Palm Beach County Metrics
- There were 505 new cases on January 17th and 5,684 new cases for the week ending January 10th (decreasing).
- The positivity rate was 9.18% for the week ending January 10th (decreasing).
- There was a decrease in COVID-like illness cases to 643 for the week ending January 10th (decreasing).
Jupiter Medical Center
- There are 31 patients currently hospitalized at JMC with COVID-19
- 5 patients are in the ICU (3 of these patients requiring ventilator support and 2 requiring BIPAP support).
- We are testing approximately 600 people per week for COVID-19 in the urgent care centers, down from 1000 per week.
Vaccine Update
Since December 23rd, Jupiter Medical Center has received approximately 2,000 doses of vaccine. Of these, roughly 1,200 doses were administered to healthcare workers with direct patient contact, comprised of JMC clinical team members and community physicians. The remaining approximately 800 doses, focusing on community members over age 65, were booked within 34 minutes of being posted on our website. 800 doses is barely enough to serve the needs of even one community in the towns surrounding JMC, let alone the over 90,000 residents over the age of 65 in our Primary Service Area (defined as the area where 75% of JMC patients reside). The limited number of doses received has simply been woefully inadequate to meet the tremendous need that exists.
As mentioned in my memo last week, recent policy changes provided hope that vaccine supply constraints would abate in February as second doses of the vaccines, which had been previously placed in reserve, would now be released immediately. Unfortunately, breaking news later in the week revealed that no stockpile exists, meaning that vaccine allocations to states for the upcoming weeks will remain flat.
State authorities are now shifting to a delivery model which will emphasize retail store chains as the primary sites for community members to receive vaccinations. Governor DeSantis yesterday announced plans to increase access sites for vaccine administration by focusing on Publix pharmacies.
We are continuously reaching out to County and State officials to request additional supply for JMC so that we can administer vaccine to our local community. We have submitted applications to AHCA (Agency for Healthcare Administration) to serve as a Community Vaccination site with the ability to administer thousands of vaccines weekly on the JMC campus and our Urgent Care Centers.
We have completely exhausted our vaccine supply and as the focus shifts away from hospitals to Publix pharmacies as vaccination sites, we do not know when or if we will receive additional shipments of vaccine. As a result, we encourage people to pursue all available avenues for receiving their vaccinations. We will continue to provide updates on our website and additional communications as we receive information from our state and county partners.
If you received your first dose of the COVID-19 vaccine at JMC, we have been assured by state authorities that we will receive an additional shipment to administer your second dose.
While early signs indicate that the most recent surge in COVID-19 cases may be receding and vaccine access sites appear to be increasing, please continue safe practices such as wearing masks and social distancing. Hopefully, the combination of the aforementioned will help slow the momentum of COVID-19 cases.
Thank you for your continued support during these challenging times.
Updated: 01/19/21
We have completely exhausted our vaccine supply. In an effort to increase vaccine access sites, State authorities announced today that vaccine administration will shift away from hospitals towards local Publix Pharmacies. As a result, we are uncertain when or if we will receive additional vaccine shipments.
To keep phone lines open for medical emergencies, please continue to check this web page for updates.
Your continued support of Jupiter Medical Center is greatly appreciated.
Updated 01/15/21
We have currently exhausted our supply of COVID-19 vaccine. We are in communication with state and local authorities to receive additional allotments. We do not know the exact timing or quantity of our next shipment. To keep phone lines open for medical emergencies, please continue to check this page for updates.
Your continued support of Jupiter Medical Center is greatly appreciated.
Updated 01/13/21
The salient updates regarding the COVID-19 pandemic are based upon the continued national surge in cases as well as a slower than expected rollout of initial vaccination administrations. New cases in the U.S. continue to rise, with over 300,000 new cases reported on January 8th. Additionally, hospitalizations continue to rise with over 129,000 patients reported in the U.S. This represents more people hospitalized now than at the peak of the spring and summer surges combined. December was the deadliest month nationally in long-term care facilities since the beginning of the pandemic. This surge has also been seen in Florida, with continued increases in the number of new cases as well as in the number of hospitalizations. The number of new cases per day has increased by 8% over the past 7 days.
The pace of vaccinations has significantly underperformed expectations. Only 9 million people have received the initial dose of 25 million vaccine doses nationally and only 600,000 individuals have received the initial dose in Florida. The biggest issue appears to be related to the supply of vaccine. In addition, getting the doses administered in a timely fashion once the vaccine has been delivered to the destination has been challenging for many hospitals, although that has not been the case for JMC.
Statewide Metrics
- There were 109,536 new cases during the week ending January 3rd.
- There were 7,713 patients hospitalized on January 12th.
- The percent positive rate averaged 12.65 for the week ending January 3rd.
- The retransmission rate (Rt) was 1.05 on January 12th.
Palm Beach County Metrics
- There were 753 new cases on January 12th and 6,113 new cases for the week ending January 3rd.
- The positivity rate was 10.85% for the week ending January 3rd.
- There was a decrease in COVID-like illness cases to 663 for the week ending January 3rd.
Jupiter Medical Center
- There are 29 patients currently hospitalized with Covid-19 at JMC.
- 7 patients are in the ICU, 2 requiring ventilators
- We continue to test over 1,000 patients per week in our urgent care centers for COVID-19.
Vaccine Update
While significant vaccine supply challenges persist, we were glad to recently receive 975 doses of the Pfizer vaccine from the state. The JMC team implemented plans to administer vaccine based on the parameters established in the governor’s executive order. Our first allotment a few weeks ago focused on healthcare workers (team members and medical staff). With many of those needs met, our most recent shipment focused on community residents over age 65. Unfortunately, due to the limited supply and unusually high demand, vaccination appointments were booked in under an hour of posting availability on the website. Approximately 75% of those appointments were booked by community members over age 65, residing mostly in Jupiter and Palm Beach Gardens with a few residing in Stuart and West Palm Beach. The remaining 25% were booked by healthcare workers from the JMC clinical team and community medical practices.
We share your frustration regarding the limited supply of the vaccine and the unpredictable nature of the process. Fortunately, policy changes over the past few days provide hope that vaccine supply constraints will abate in the upcoming weeks. Starting in two weeks, vaccines will be distributed weekly to states based on the number of residents over 65-years old and by the pace of administration in each state. Furthermore, second doses of the vaccines, which had been previously placed in reserve, will now be released immediately. These changes should help us secure more vaccine in the upcoming weeks. While this may cause concern for those who have been fortunate to receive the first dose of the vaccine, we have been assured by state officials that current vaccine production rates will be sufficient to meet second dose requirements. Florida State Surgeon General, Dr. Scott Rivkees, testified before the State Healthcare Committee this morning that our state is scheduled to receive 250,000 vaccine doses weekly, exclusive of the second dose supply. Dr. Rivkees also testified that the CDC has committed to having the necessary doses for the second shot.
Further encouraging news came from Moderna’s Chief Medical Officer earlier this week at JP Morgan’s 39th Annual Healthcare Conference. Moderna believes that its vaccine should provide protection against COVID-19 for at least a year. This is an important development since current vaccine administration projections delineate a 9-12 month timeline for vaccine administration across the U.S. If a booster dose were to be required within this timeframe, it would pose further logistical challenges.
Based on the aforementioned information, we are optimistic that vaccine supply will improve in February. We continue to advocate with state and local officials for larger allocations of vaccine for JMC in upcoming shipments and have applied as a Community Vaccination site. We realize that this is a trying process as COVID-19 cases rise and vaccine distribution seems haphazard at best.
Please know that the JMC team is working diligently to obtain as much vaccine as possible, as quickly as possible, in order to meet the needs of our community and healthcare workers.
Thank you for your support during these challenging times.
Update 01/12/21
We shared here yesterday that we received a limited supply of the Pfizer COVID-19 vaccine for community members over age 65 and healthcare workers. The demand was very high and available appointments filled quickly. We anticipate that as vaccine supply becomes available, we will be able to offer more appointment times to our community.
We are working closely with the state of Florida to receive additional allotments of the vaccine. Please continue to monitor the website for updates. In the interim, should you wish to pursue vaccination through another avenue, we encourage you to do so to avoid any delays.
We look forward to serving you in the future.
Updated 01/05/21
A Message from the CEO, Amit Rastogi, MD, MHCM
The most significant developments related to the COVID-19 pandemic are the expected surge related to the Christmas/New Year’s holidays, the detection of the UK variant B117 in the United States and the rollout of the Pfizer/ Moderna vaccines. CDC forecasts project a sustained high level of 1.2 to 2 million new cases per week in the U.S. National models continue to project an increasing number of hospitalizations due to COVID-19. State level models demonstrate a continued high level of new cases in Florida (75,000 new cases per week) over the next 4 weeks. Unfortunately, we are experiencing the impact of this trend on our urgent care centers, emergency department and our inpatient wards/ICU, as demonstrated by the JMC metrics below.
The second important item is the detection of the UK variant in the US. The B117 variant is believed to be 40-70% more transmissible than other strains, meaning that more persons are likely to be infected when they come in contact with an infected individual. Projections show that the retransmission rate (Rt) may be increased by 0.4 to 0.7 by the new strain. It is not yet known whether the new strain produces more severe disease. Scientists are currently evaluating the susceptibility of the variant strain to the current vaccines. At the present time, it is believed that the Pfizer and Moderna vaccines will be effective against the new strain.
Statewide Metrics
- There were 91,109 new cases during the week ending December 27th
- There were 7,245 patients hospitalized on January 3rd
- The percent positive rate averaged 12.4% for the week ending December 27th
- The retransmission rate (Rt) is 1.15
Palm Beach County Metrics
- There were 796 new cases on January 4th and 5,088 new cases for the week ending December 27th.
- The positivity rate was 9.85% for the week ending December 27th.
- COVID-like illness cases have increased to 617 new cases per week (ending December 27th).
Jupiter Medical Center Metrics
- There are 29 COVID positive patients admitted at JMC.
- 6 of these patients are in the ICU (two patients requiring mechanical ventilation, one requiring BIPAP support).
- The number of patients being tested daily in the ED for COVID illness has jumped to over 50 per day.
- We are testing over 1,000 patients per week in our urgent care centers for COVID.
Vaccines
While the development of the Pfizer and Moderna vaccines in record time has been a boon, distribution of the vaccines has proven to be a significant logistical challenge around the globe and our nation.
In Florida, the state has received nearly 965,000 doses of vaccines for Covid-19, of which 533,000 doses have been provided to hospitals and approximately 250,000 of those vaccines have been administered. The vast majority of the doses arrived between 12/22/20 and 12/29/20, hampering rollout due to the holiday break.
On December 23, 2020, Governor Ron DeSantis signed an Executive Order prioritizing vaccine administration to healthcare workers, long-term care facility residents and those over age 65. Unfortunately, the current supply of vaccine is not enough to meet the demand even for the priority categories, and it will require months to successfully vaccinate these individuals. Hospital staff and long-term care residents/staff in Florida constitute at least 750,000 individuals and there are more than 4.5 million individuals 65 and older. Compounding this challenge is the fact that the current vaccines require administration of a second dose to these recipients within 3-4 weeks of the first one.
The first week of Covid-19 vaccine deployment in Florida began with the distribution of Pfizer vaccine to five pilot sites, for the purpose of vaccinating healthcare workers and long-term care residents. Week two marked shipment of the Moderna vaccine to hospitals across the state for healthcare workers, along with vaccination of long-term care residents by CVS/Walgreens. In week three, all 67 county health departments received the vaccine to begin community vaccinations.
As we enter week four, Governor DeSantis has directed the Florida Division of Emergency Management to work with the Florida Department of Health to identify state-run COVID-19 testing sites that can be converted into vaccine sites. He has also directed the Florida Division of Emergency Management to identify places of worship and other locations in underserved communities where the vaccine may be administered. Florida has already begun a pilot program in Escambia County, where over
500 seniors have received their vaccine and have been scheduled for their booster shot, which they will receive at the same location. The governor has directed the Florida Division of Emergency Management to hire 1,000 contract nurses to support vaccination efforts and these nurses will be deployed throughout the entire state to help run vaccination sites. Governor DeSantis is also directing the Florida Division of Emergency Management to assume additional responsibilities regarding the administration of vaccines in Florida’s over 3,000 assisted living facilities, supplementing and accelerating the efforts being undertaken by CVS and Walgreens, pursuant to their agreement with the federal government. At this time, vaccines for those over 65 will be available from the county health department and hospitals.
At Jupiter Medical Center, we are proud that we were able to start vaccinating healthcare workers (team members and medical staff) within 2 hours of receiving our first vaccine allocation on December 23rd. Additionally, we administered the vaccine on Christmas eve as well as Saturday and Sunday of the holiday weekend to increase access for medical staff providers unable to participate during the week due to patient care responsibilities.
We have exhausted our first allocation of the vaccine focusing on healthcare workers and have submitted a plan to the Agency for Healthcare Administration (AHCA) requesting a significant increase in future allotments. This would allow us to vaccinate thousands of community members weekly, prioritizing those over 65 years of age, while continuing to vaccinate healthcare workers. We have offered to conduct community vaccination clinics on JMC campus, our Urgent Care Center locations and in the community, seven days a week.
While we don’t yet know the exact timing or quantity of our next shipment of vaccine from the state, we anticipate receiving it as early as next week. Much of the uncertainty stems from a national vaccine supply chain process which is still under development. Currently, the states receive a varying allotment of vaccine on a weekly basis from federal authorities and subsequently allocate it to hospitals and county health departments. In our discussions with state health agency officials, they have assured us that significant efforts are underway to streamline this process and improve predictability of vaccine allotment in the upcoming weeks and months.
JMC leadership continues to advocate with state and local leadership/legislators for increased vaccine allocation for our community. As mentioned above, we have a plan in place to administer community vaccinations, which we are ready to operationalize as soon as we receive notice of our next vaccine allocation. Once we receive notification, we will send out detailed communication regarding the appointment scheduling process, locations, etc.
While we are all glad to put 2020 behind us, we face significant challenges for the first few months of 2021. COVID-19 cases are on the rise and are projected to increase over the next 4-6 weeks as a result of holiday travel. Although vaccines will provide much needed protection against future spread of the virus, the current demand far outpaces supply.
Please know that through these trying times, the entire JMC team is here to serve. Whether it is to diagnose those presenting with COVID-19 symptoms at our urgent care centers/emergency department, or to provide the latest treatments for those requiring admission to the hospital, we are here. While the vaccine rollout presents significant challenges, we are advocating vigorously to obtain as many doses as possible for community vaccinations and re-purposing personnel throughout the organization to operationalize vaccine clinics as soon as we receive more vaccine.
We will get through these trying times together.
Best wishes for a safe and healthy new year.
Updated 12/29/20
A Message from the CEO, Amit Rastogi, MD, MHCM
JMC received its first shipment of the Moderna Covid-19 vaccine from the Florida Department of Health on Wednesday, 12/23/20. I am proud to say that we began administering it to our healthcare workers (team members/medical staff) within 90 minutes of arrival.
In just 6 days, we have vaccinated over 625 members of our staff, physicians and nurses, prioritizing those who face high risk exposures or high frequency of events. While we continue to vaccinate our healthcare workers, we are working closely with the Florida Department of Health and anticipate vaccinating community members, 65 and older, in the upcoming weeks based on CDC recommendations and the Governor’s Executive Order. To keep you informed, we will continue to post updates on Tuesday and Friday.
Please remember, your best protection from #COVID19 will be a combination of getting a COVID-19 #vaccine, wearing a mask, staying at least 6 feet away from others, avoiding crowds, and washing your hands often.
Statewide Metrics
-
There were 68,614 new cases during the week ending December 20th
-
There were 6,400 patients hospitalized on December 28th
-
The percent positive rate averaged 9.03% for the week ending December 20th
-
The retransmission rate (Rt) is 1.05
Palm Beach County Metrics
-
There were 626 new cases on December 28th and 3,529 new cases for the week ending December 20th
-
The positivity rate was 7.15% for the week ending December 20th
-
COVID-like illness cases have remained stable at 472 new cases per week (ending December 20th)
Jupiter Medical Center Metrics
-
There are 21 COVID positive patients admitted at JMC
-
5 of these patients are in the ICU (one patient requires BIPAP respiratory support)
-
There has been a 4% increase in the number of patients seen in the Urgent Care Centers for COVID testing.
Updated 12/23/20
A Message from the CEO Amit Rastogi, MD, MHCM
The surge in new Covid-19 cases and hospitalizations has continued over the past several weeks, partly a sequela of the Thanksgiving holiday. On December 18th, there were over 400,000 new cases in the U.S. There are over 100,000 patients hospitalized in the U.S. and 21,000 requiring ICU level care. Current models project a continued increase in cases over the next 4 weeks and predict 1.2 to 2.3 million new cases will be reported in the week ending January 9th. On a positive note for our state, the consensus projection for models of Florida and Palm Beach County demonstrate a plateau in number of new cases over the next 4 weeks. The state is projected to add 75,000 new cases weekly over the next 4 weeks.
A concerning new development is the emergence of a more transmissible strain of COVID in the United Kingdom. The new variant was first detected in September and already accounts for two-thirds of cases in London. The new strain also exhibits mutations involving the spike protein, which is worrisome because current vaccines target the spike protein. Scientists are studying whether the current vaccines will be effective against the new variant and they believe that will be the case. Due to these concerns, there has been a rapid move to limit travel from the UK to slow the spread of the new variant.
Statewide Metrics
- There were 74,545 new cases during the week ending December 13th
- There were 5,241 patients hospitalized on December 21st
- The percent positive rate averaged 9.38% for the week ending December 13th
- The retransmission rate (Rt) is 1.08
Palm Beach County Metrics
- There were 459 new cases on December 20th and 3,791 new cases for the week ending December 13th
- The positivity rate was 7.59% for the week ending December 13th
- COVID-like illness cases have decreased to 470 new cases per week (ending December 13th)
Jupiter Medical Center Metrics
- There are 20 COVID positive patients admitted at JMC
- 2 of these patients are in the ICU (both patients require BIPAP respiratory support)
- We are testing approximately 25 patients per day in the ED for COVID-19
- There has been a 30% increase in the number of patients seen in the Urgent Care Centers for COVID testing.
Vaccines
We are proud to report that JMC received its first shipment of the Moderna Covid-19 vaccine today from the state and we have initiated administration of the vaccine for healthcare workers, a very significant advance in our battle against the pandemic. An FDA advisory panel recommended on Thursday, December 17th that the Moderna vaccine receive an Emergency Use Authorization (EUA), which was issued this past Friday.
The Moderna vaccine is based on a messenger RNA platform. The messenger RNA enters the cell and encodes the spike protein of the virus, which in turn causes the body to develop antibodies against this antigen. The vaccine demonstrated 94.1% efficacy in preventing COVID infection in a Phase III trial which included 30,000 participants. Vaccination requires 2 doses, 4 weeks apart. Additionally, there were no severe cases of COVID disease in the few patients who did contract COVID illness after vaccination. The side effect profile is excellent. There were no patients who had an anaphylactic or severe hypersensitivity reaction. The most common adverse reaction was injection site pain (90%). A flu-like syndrome with: fatigue (60%), muscle pain (60%), joint pain (40%), and chills (40%) which lasted for 1 to 2 days was also fairly common. This syndrome was more frequently seen after the 2nd dose and in younger patients (<55 years old). Serious adverse events occurred in less than 1% of patients in the vaccine group and in the control group, with no evidence that these were related to the vaccine.
On December 20, 2020 the CDC’s Advisory Committee on Immunization Practices (ACIP) released its recommendations regarding COVID-19 vaccine administration. Their goal is to balance the prevention of morbidity/mortality from COVID with the preservation of societal functioning:
Frontline Essential Workers are defined as workers who are in sectors essential to the functioning of society and are at substantially higher risk of exposure to COVID (i.e. firefighters, police, teachers, etc.) while Other Essential Workers include those in transportation, food services, housing, etc.
While CDC data reveals that COVID-19 incidence is highest in young adults (ages 18-29), mortality rates are highest in older adults (those over 85 years followed by ages 75-84).
From a population perspective, group 1a represents approximately 24 million people, group 1b about 49 million unique persons and group 1c represents 129 million unique persons. Barring unforeseen circumstances, CDC models predict requiring roughly 8 weeks to vaccinate group 1a, 12 weeks for group 1b and 24 weeks for group 1c.
In Florida, the Department of Health is partnering with pharmacies (CVS, Walgreens, etc.) to administer COVID vaccinations for long term care facilities. Therefore, JMC’s initial vaccination plan focuses on healthcare workers. Based on CDC guidelines, our leadership team has developed a plan for vaccinating JMC team members and our medical staff, prioritizing vaccinations for those who face high risk exposures or high frequency of events. This includes individuals working in our COVID Unit, the Emergency Department, Intensive Care Unit, etc.
In line with national models, we anticipate that it will take several weeks to provide the initial dose of the vaccine to our team members and medical staff. We will be working with state authorities to coordinate JMC participation in the vaccination of our community members, which we anticipate will begin in the spring based on CDC projections. This plan continues to evolve and the timeframe depends on our allocation of vaccine, when they are received, as well as evolving CDC and ACIP recommendations.
We remain committed to being a leader in the fight against the pandemic and coordinating with state and local authorities to rapidly vaccinate our team members, providers, and members of our community. In an effort to keep you apprised of this rapidly evolving situation, which affects all of us and our families, we will update our website every Tuesday and Friday and post details as they become available via the following link: https://www.jupitermed.com/covid19-vaccine/.
The arrival of the Covid-19 vaccine certainly brings much needed hope that we will all soon be on the path to “normalcy’ in our lives for which we have been yearning.
Best wishes for a happy and safe holiday season for you and your families.
-
Jupiter Medical Center
We want to help you! If you have questions about our services and what we can offer you and your loved ones, please reach out.


